ZYRTEC®
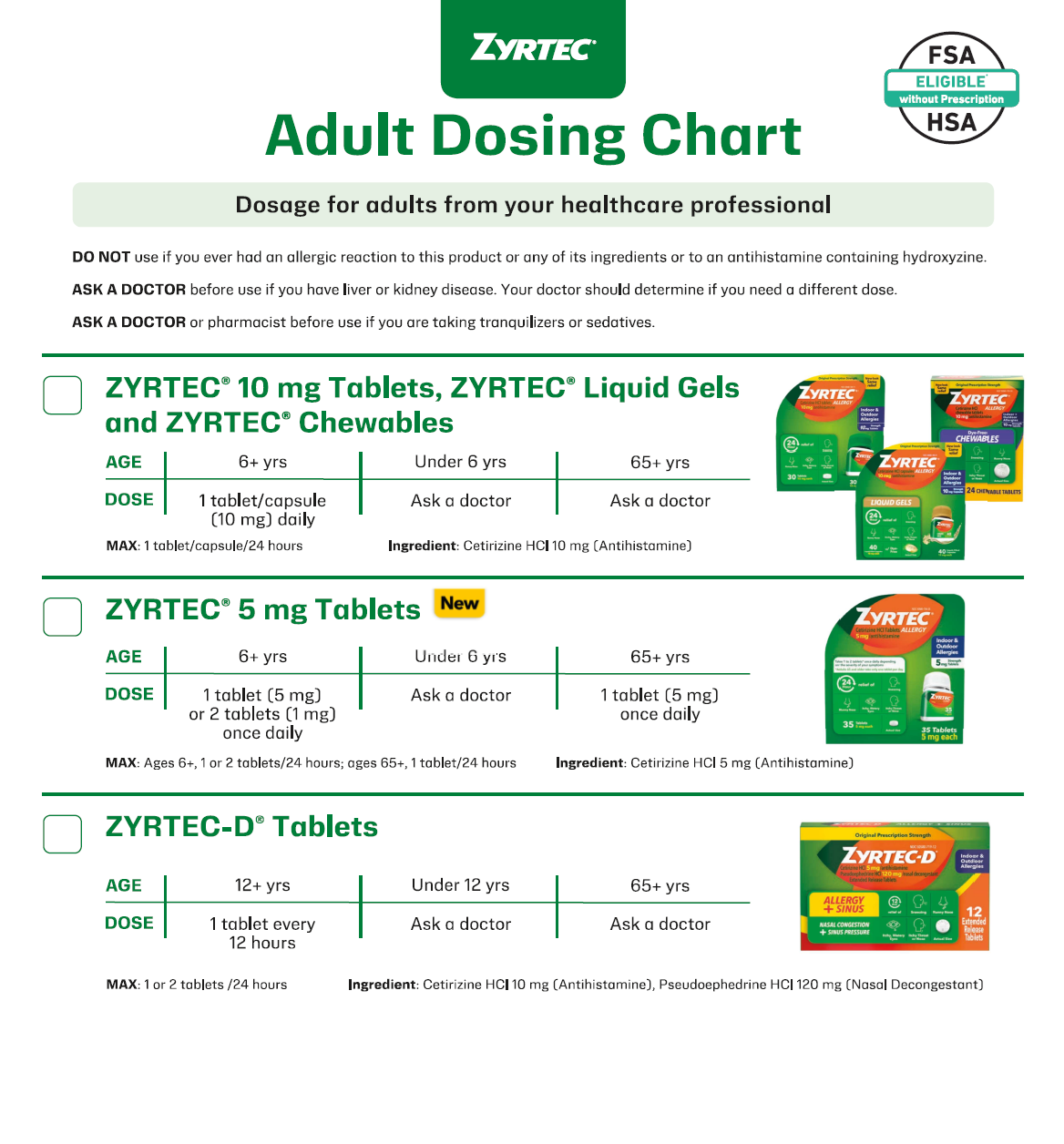
Start with ZYRTEC® for allergy relief,* whether patients’ symptoms are mild, moderate, or severe
What's New
Join us at these upcoming conferences:
FEBRUARY 27 - MARCH 2, 2026 - PHILADELPHIA, PA, USA
Join Us at the AAAAI 2026 Conference!
ZYRTEC® will be at Booth #1026
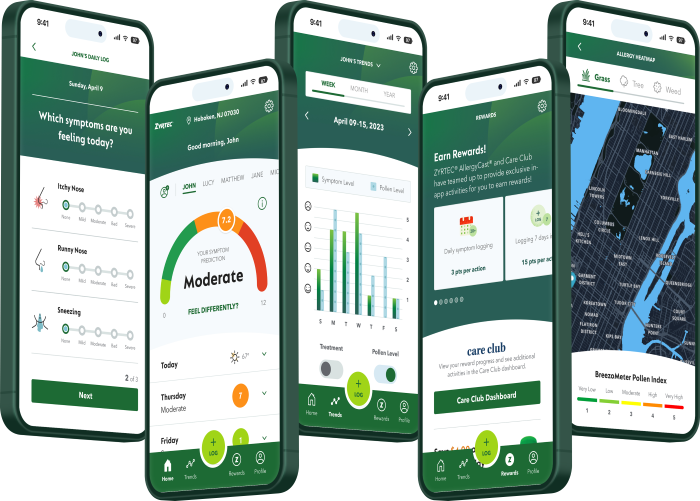
Equip Your Patients with Management Tools!
The ZYRTEC® AllergyCast® app is a free tool that helps your patients manage their allergies effectively. By leveraging local data on pollen counts and air quality, it provides personalized daily allergy impact scores to predict symptom severity. Patients can receive tailored insights and access helpful tips and exclusive savings on ZYRTEC® products.
Featured products
Watch the Chronic Spontaneous Urticaria (CSU) Webinar
In this webinar, Dr. Friedman will discuss the diagnosis and classification of Chronic Spontaneous Urticaria, Dr. Gupta will address the disease burden and patient case, and Nurse Julia will focus on CSU management.
Order allergy treatment samples for your patients
Get free samples of popular products from ZYRTEC®.
*ZYRTEC® relieves sneezing; runny nose; itchy, watery eyes; and itching of the nose or throat.
†ZYRTEC® 10 mg starts working at hour 1 and Claritin® starts working at hour 3, based on first dose on the first day of a 2-day study in 2 pollen chamber studies. Primary endpoint measured mean improvement from baseline in Major Symptom Complex (MSC) severity score. MSC symptoms included runny nose, sniffles, itchy nose, nose blows, sneezes, and watery eyes.
‡Based on first dose on the first day of a 2-day pollen chamber study with ZYRTEC® 10 mg vs Allegra® 180 mg at hours 21 to 24. Primary efficacy endpoint was change in total symptom severity score from baseline at hours 21 to 24. Total Symptom Severity Complex score was defined as the sum of self-assessed severity scores of 4 symptoms: runny nose, sneezing, itchy nose/palate/throat, and itchy/watery eyes.
References: 1. Day JH, Briscoe M, Widlitz MD. Cetirizine, loratadine, or placebo in subjects with seasonal allergic rhinitis: effects after controlled ragweed pollen challenge in an environmental exposure unit. J Allergy Clin Immunol. 1998;101(5):638-645. 2. Day JH, Briscoe M, Rafeiro E, Chapman D, Kramer B. Comparative onset of action and symptom relief with cetirizine, loratadine, or placebo in an environmental exposure unit in subjects with seasonal allergic rhinitis: confirmation of a test system. Ann Allergy Asthma Immunol. 2001;87(6):474-481. 3. Patel M, Urdaneta E, Franklin K, et al. Cetirizine significantly relieves ocular allergy symptoms in subjects with seasonal allergic rhinitis. Poster Abstract. Data on file, Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division. 4. Day JH, Briscoe MP, Rafeiro E, Hewlett D, Chapman D, Kramer B. Randomized double-blind comparison of cetirizine and fexofenadine after pollen challenge in the environmental exposure unit: duration of effect in subjects with seasonal allergic rhinitis. Allergy Asthma Proc. 2004;25(1):59-68.