Seasonal Allergic Rhinitis (SAR)
ZYRTEC® Provides Consistent Symptom Relief From SAR Over a 2- to 4-Week Treatment Period1*
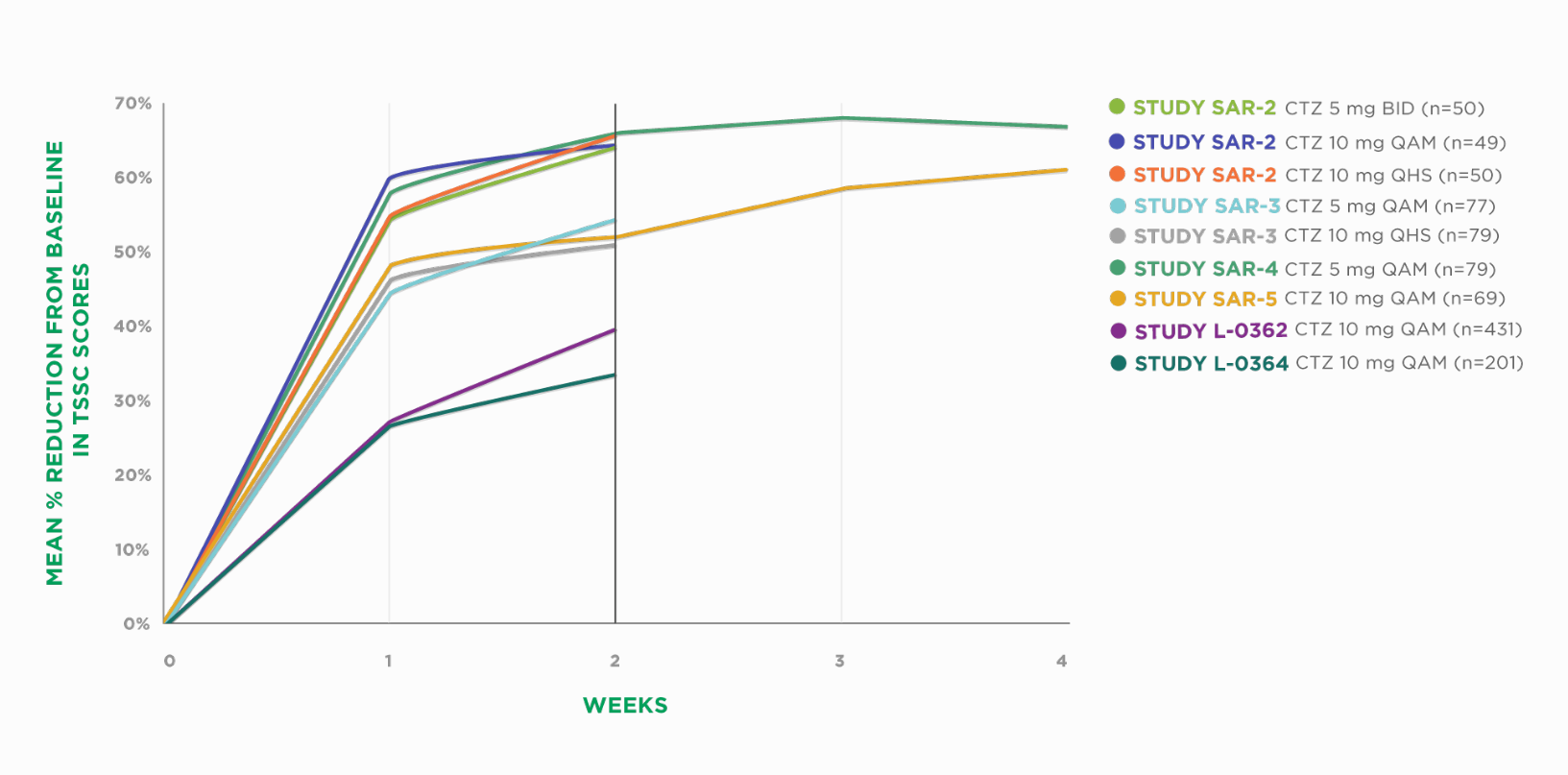
BID=twice daily | CTZ=cetirizine | QAM=every morning | QHS=every night | TSSC=Total Symptom Severity Complex
Ocular Symptoms
Itchy, watery eyes affect patients’ quality of life.4,5 ZYRTEC® provides proven symptom relief2
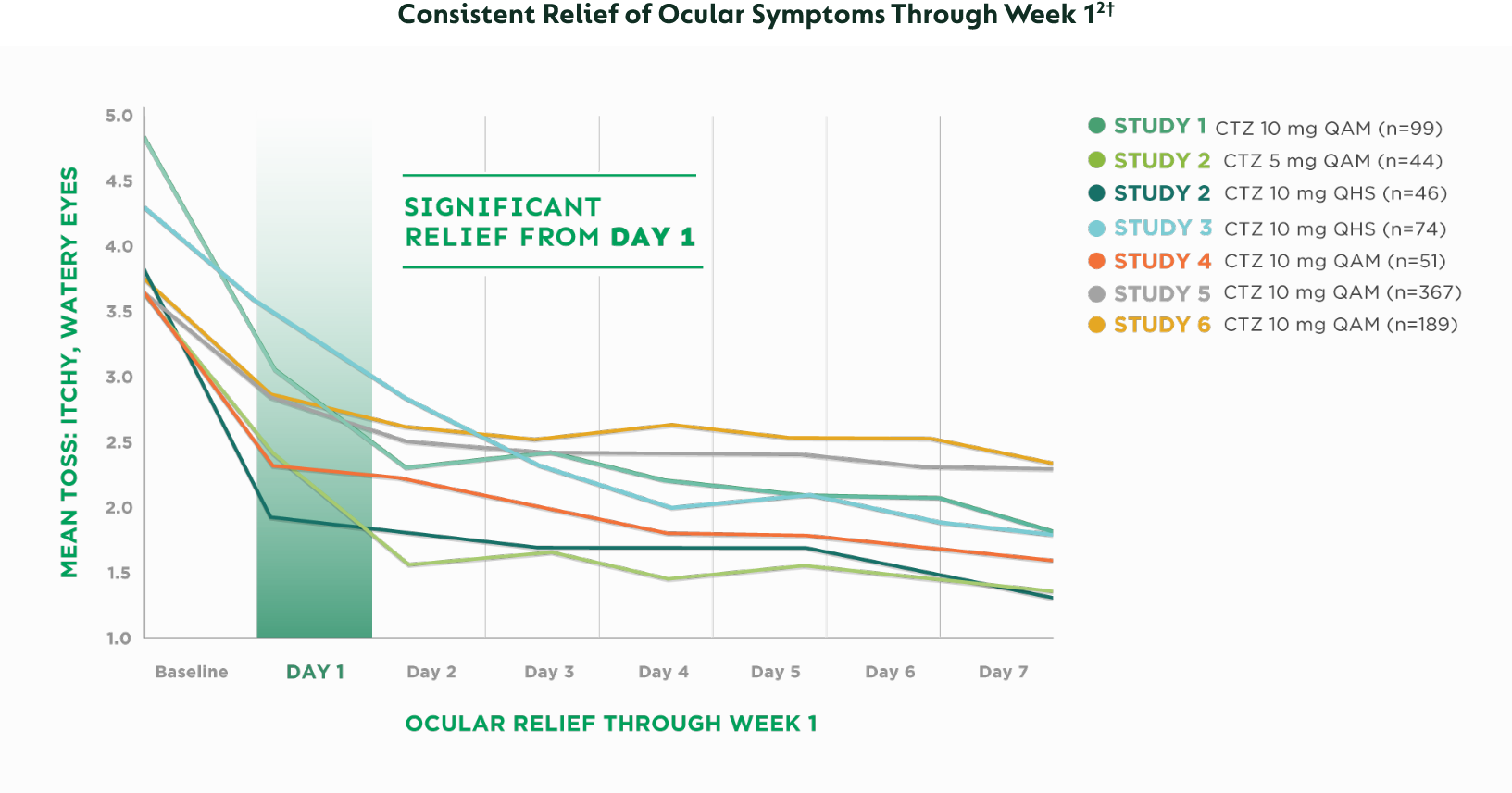
CTZ=cetirizine | QAM=every morning | QHS=every night | TOSS=Total Ocular Symptom Score
Improvement in Total Ocular Symptom Score (TOSS) during the first week of treatment†
Large multicenter study of 1255 seasonal allergy patients, most with moderate/severe symptoms based on a 4-point scale (0=none to 3=severe)
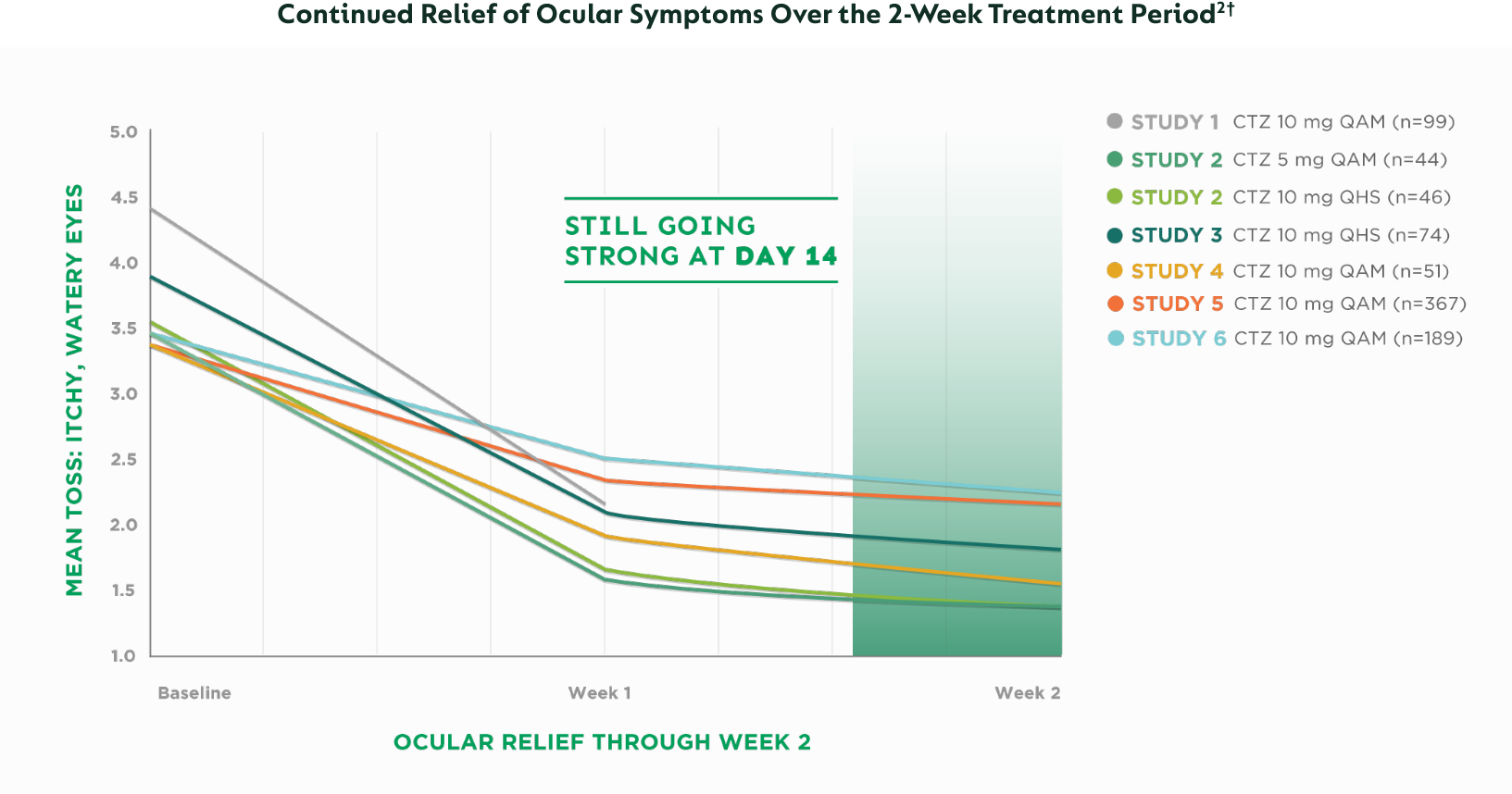
CTZ=cetirizine | QAM=every morning | QHS=every night | TOSS=Total Ocular Symptom Score
2-week data: 35% to 62% mean improvement from baseline in ocular scores†
Up to 80% of people with allergic rhinitis have ocular symptoms,6 which can lead to lower quality of life and reduced work productivity, including impairment while working and a significant impact on work hours missed.4,5
Nasal Symptoms
Powerful relief of sneezing, runny nose, and itchy nose3
Relief of Nasal Symptoms Through Week 13‡
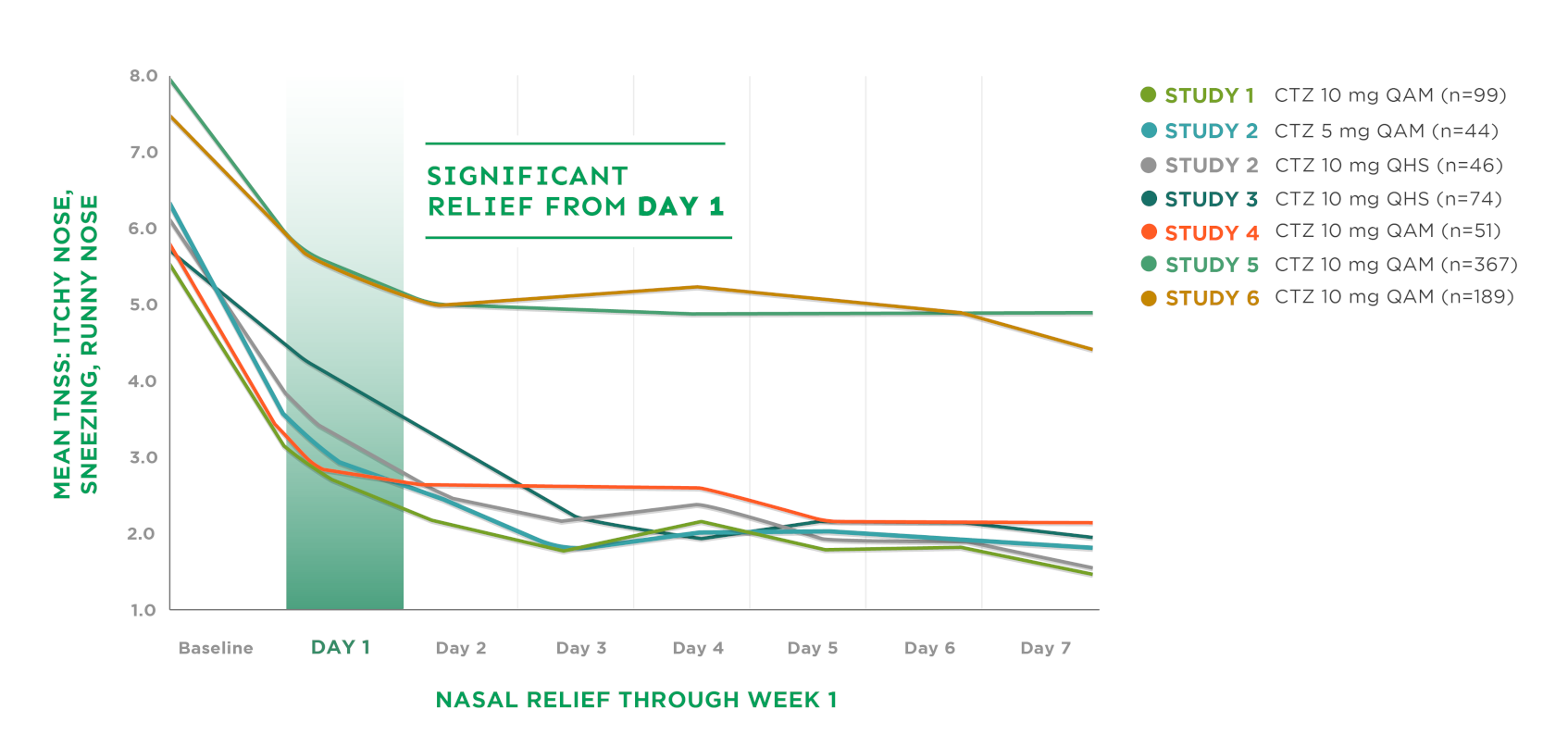
CTZ=cetirizine | QAM=every morning | QHS=every night | TNSS=Total Nasal Symptom Score
Large multicenter study included 921 patients with SAR, most with moderate/severe symptoms based on a 4-point scale (0=none to 3=severe)
Improvement demonstrated in the first week of treatment‡
Significant improvement continued over the 2-week treatment period, with 29% to 63% mean improvement from baseline in nasal severity scores‡
*All studies are multicenter, randomized, placebo-controlled, double-blind, minimum 100 participants with previous diagnosis of SAR, and adequate minimum duration (2 weeks). Total symptom severity score measured by patient.
†Randomized, placebo-controlled studies of SAR patients (N=1255). Total ocular symptom score (TOSS) was the sum of the severity scores for itchy eyes and watery eyes; one study also included red eyes. Individual symptom scores were rated on a 4-point scale (0=none to 3=severe). SAR 1 was 1 week in duration.
‡Randomized, placebo-controlled studies of SAR patients (N=921). Total Nasal Symptom Score (TNSS) was the sum of the severity scores for sneezing, runny nose, itchy nose in all studies; 2 studies included post-nasal discharge. Individual symptom scores were rated on a 4-point scale (0=none to 3=severe). SAR 1 was 1 week in duration. Changes from baseline over a 2-week period, by day in the first week, and weekly were assessed.
References: 1. Data on file, Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division. 2. Patel M, Urdaneta E, Franklin K, et al. Cetirizine significantly relieves ocular allergy symptoms in subjects with seasonal allergic rhinitis. Poster abstract. Data on file, Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division. 3. Urdaneta E, Tian X, Wu M, et al. Cetirizine effectively relieves both ocular allergy symptoms and nasal allergy symptoms in subjects with seasonal allergic rhinitis [abstract]. J Allergy Clin Immunol. 2014;133(suppl 2):AB279. Accessed January 27, 2023. https://www.jacionline.org/article/S0091-6749(13)02892-3/abstract 4. Virchow JC, Kay S, Demoly P, Mullol J, Canonica W, Higgins V. Impact of ocular symptoms on quality of life (QoL), work productivity and resource utilisation in allergic rhinitis patients - an observational, cross sectional study in four countries in Europe. J Med Econ. 2011;14(3):305-314. 5. Palmares J, Delgado L, Cidade M, Quadrado MJ, Filipe HP, Season Study Group. Allergic conjunctivitis: a national cross-sectional study of clinical characteristics and quality of life. Eur J Ophthalmol. 2010;20(2):257-264. 6. Kari O, Saari KM. Diagnostics and new developments in the treatment of ocular allergies. Curr Allergy Asthma Rep. 2012;12(3):232-9.
