Nicorette® (nicotine)
8/10 smokers and 6/10 vapers want to reduce or quit1. Help your patients quit with Nicorette®.
Click here for product information.

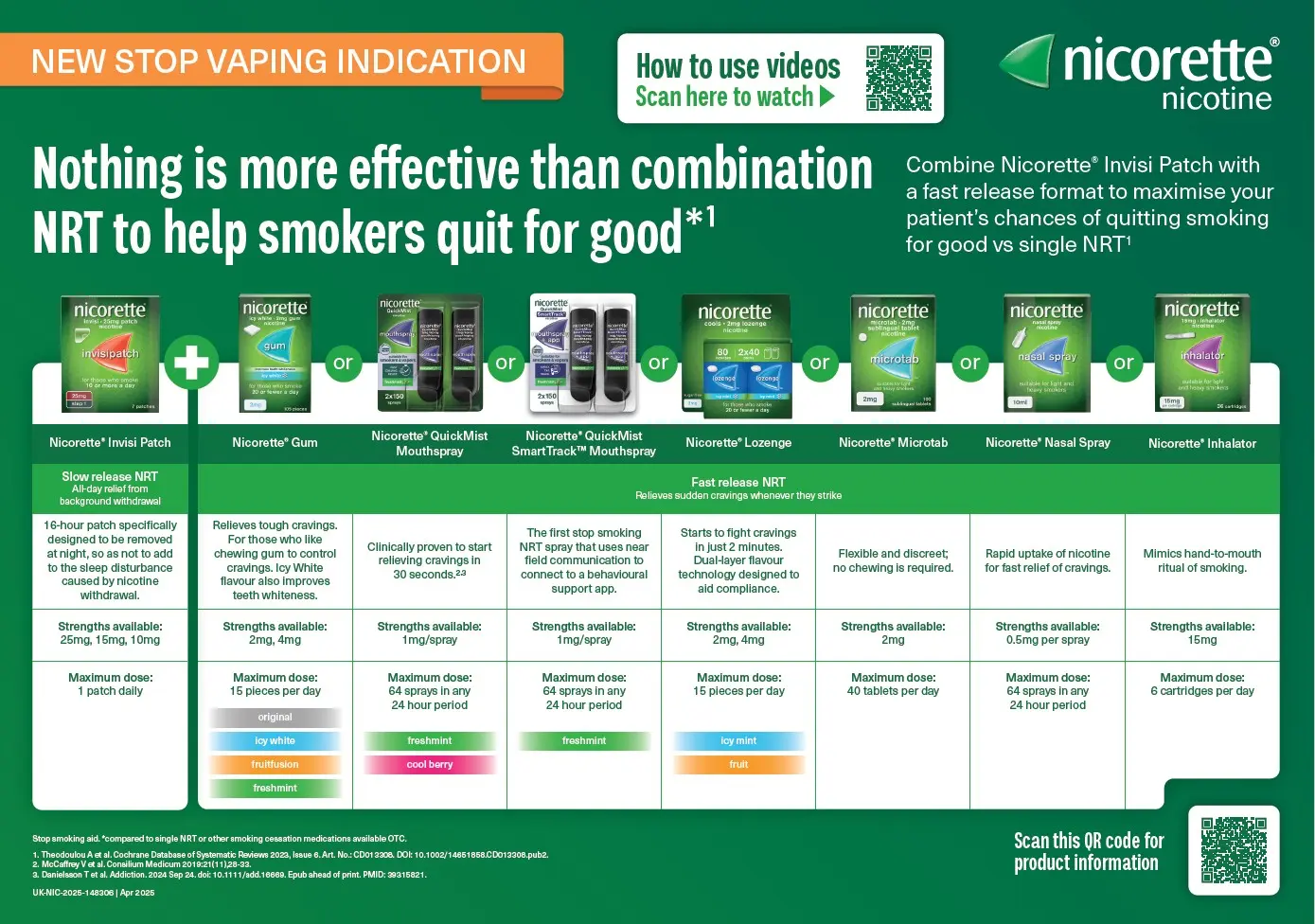
Discover why combination Nicotine Replacement Therapy (NRT) is right for your patients to help them quit smoking
Help improve your patient's chances of quitting smoking* by utilizing Nicorette combination therapy. This involves combining a long-acting nicotine patch, delivering nicotine throughout the day to help combat withdrawal symptoms, and a faster acting NRT delivery format to use if cravings strike. This approach offers a simple and flexible dosing schedule, improving effectiveness vs single NRT, particularly for patients with high nicotine dependence or who have tried NRT before.2
*vs NRT monotherapy
Nicorette® is designed to help your patients become smoke and nicotine free*Δ
Backed by 45+ years of real-world experience and over 240+ clinical trials3,4
Combination of Nicotine Replacement Therapy (NRT) is 27% more effective than single NRT2
Nicorette QuickMist starts to reduce cravings in 30 seconds5
Provides effective craving relief6-12
Many clinical guidelines globally recommend NRT as a first line treatment for smoking cessation therapy13-15
On the WHO list of essential medicines16
* Abstinence rates: Combination Therapy (nicotine patch + active gum): 27.5% vs nicotine patch + placebo gum: 15.3%.17
Δ In smokers, even from Nicorette itself.
Nicorette® NRT solutions6-12
Visit our consumer site
References
Data on file. Incite 2024. Kenvue Global CEPs Usage and Attitude Report.
Theodoulou, A et al. 2023. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews, (6).
Ferno O. Addiction 1994;89:1215-1226
Kenvue internal data
McAffrey V et al. Consilium Medicum 2019;21(11):28-33. Danielsson T et al. Addiction. 2025 Jan;120(1):95-105. doi: 10.1111/add.16669. Epub 2024 Sep 24. PMID: 39315821; PMCID: PMC11638496.
Nicorette® QuickMist. Summary of Product Characteristics.
Nicorette® Invisi 25 mg Patch. Summary of Product Characteristics
Nicorette® 4mg Gum Summary of Product Characteristics.
Nicorette® Cools 4mg Lozenge Summary of Product Characteristics.
Nicorette® 15mg inhalator. Summary of Product Characteristics.
Nicorette® Nasal Spray Summary of Product Characteristics.
Nicorette® 2mg Microtab Summary of Product Characteristics.
Canada Alberta Health Services Tobacco, Vaping and Cannabis Program – June 2021.
World Health Organisation, Model List of Essential Medicines, 23rd list 2023, WHO Model List of Essential Medicines – 23rd list, 2023.
NICE guidance. Tobacco: preventing uptake, promoting quitting and treating dependence [NG209] Nov 2021.
WHO. A Guide for Tobacco Users to Quit. https://www.who.int/publications/i/item/9241506939
Kornitzer M, et al. Prev Med. 1995;24(1):41–47.
UK-NIC-2025-241046 | October 2025