Cosmetic vs therapeutic mouthrinses
With mouthrinse becoming an accepted part of a patients' daily oral health regimen, they must understand the fundamental differences between cosmetic and therapeutic mouthrinses.
Antimicrobial mouthrinses on the market today have 3 main ingredients. One is called quaternary ammonium compound cetylpyridinium chloride, or CPC, an antiseptic that has been used since 1939 and is still included in many current mouthrinses at formulations ranging from 0.045-0.075%. It's important to note that 0.045% is the minimum recognized therapeutic concentration; yet, many mouthrinses contain CPC at concentrations below this and do not provide anti-plaque/anti-gingivitis benefits.
A second common antimicrobial ingredient found in mouthrinses is chlorhexidine, or CHX, a bacterial bisbiguanide antiseptic that is found only in prescription products such as Peridex™ and PerioGuard®.
CPC can interact with negatively charged ions in toothpaste, which may lower its biological activity and reduce its clinical efficacy.2 CPC and CHX also may precipitate negatively charged dietary chromogens in food and beverages, which can lead to extrinsic staining.3-5
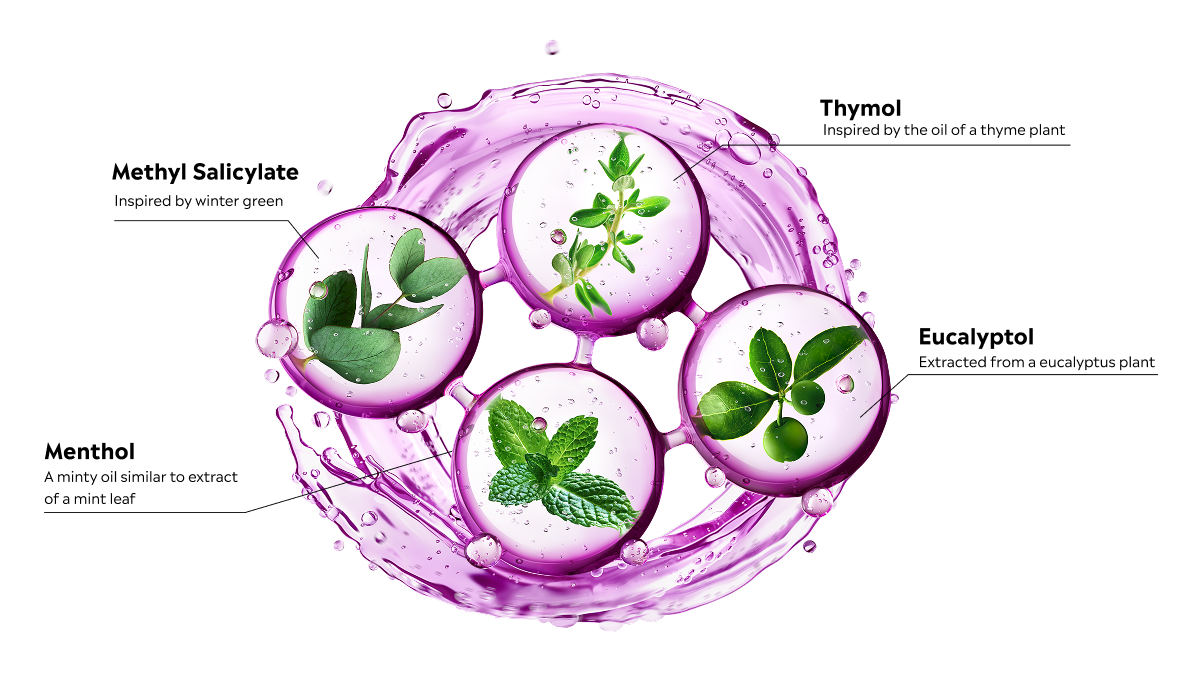
Finally, some over-the-counter (OTC) mouthrinses contain a fixed combination of the 4 Essential Oils eucalyptol, menthol, methyl salicylate, and thymol, the ingredients found in all types of LISTERINE® Antiseptic. This unique combination of ingredients is specifically designed to deeply penetrate biofilm and kill bacteria that can lead to gingivitis.
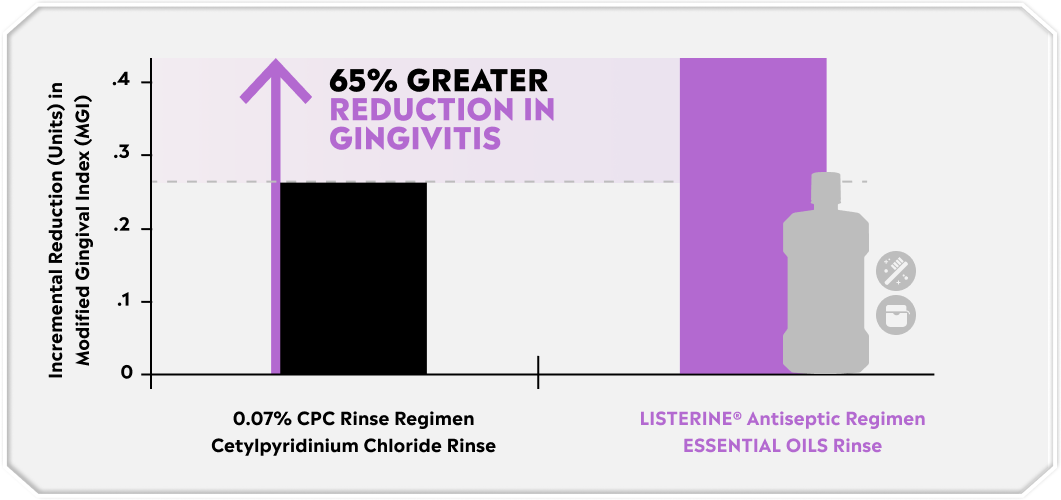
The LISTERINE® mouthrinse difference: deeper biofilm penetration in 30 seconds.
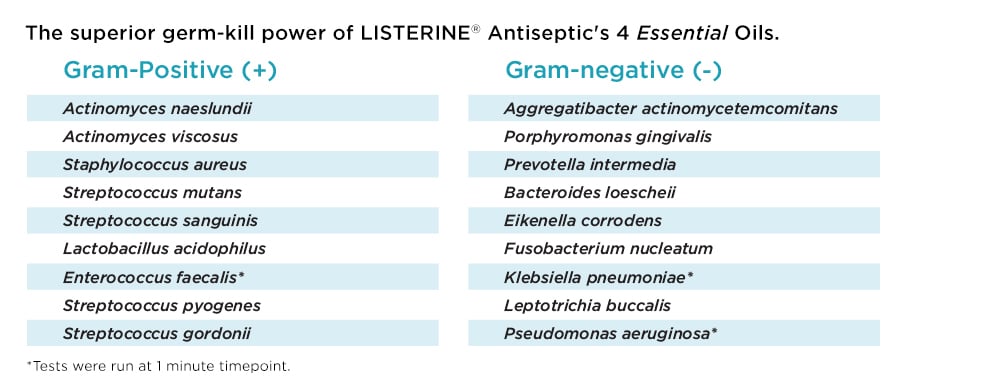
With the help of our unique 4 Essential Oils formulation, LISTERINE® Antiseptic penetrates biofilm, killing bacteria in virtually 100% of the mouth.2,6-8 Plus, it lyses, kills, and inhibits the growth of both gram-positive and gram-negative bacteria.8-14
To learn more about the bacteria-killing power and mechanism of action built into LISTERINE® Antiseptic products through the unique 4 Essential Oils formulation, watch our video. LISTERINE® Antiseptic's 4 Essential Oils are also shown to penetrate plaque biofilm deeper than CPC.15* Take a look at a comparison between the tooth surface of patients using it and patients using CPC. The image shows living bacteria cells (green) and dead bacteria cells (red) at the deepest layer of biofilm As you can see, LISTERINE® Antiseptic killed almost twice as many bacteria than CPC.15
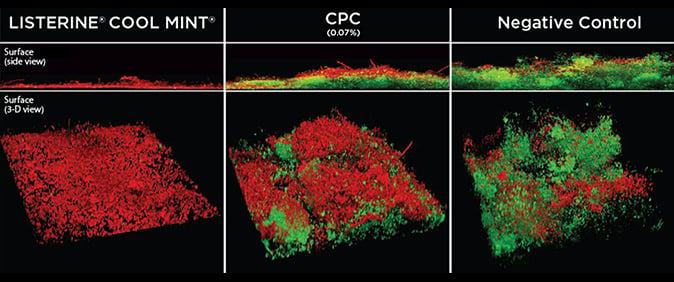
The clinical difference: LISTERINE® mouthrinse vs. other mouthrinses.
It is particularly notable how well LISTERINE® Antiseptic has performed when studied against other mouthrinses, including prescription-only Peridex™. When compared side-by-side as adjuncts to proper oral hygiene home care measures in reducing supragingival dental plaque and gingivitis, LISTERINE® Antiseptic produced gingivitis reductions comparable to Peridex™ at 6 months.15,16
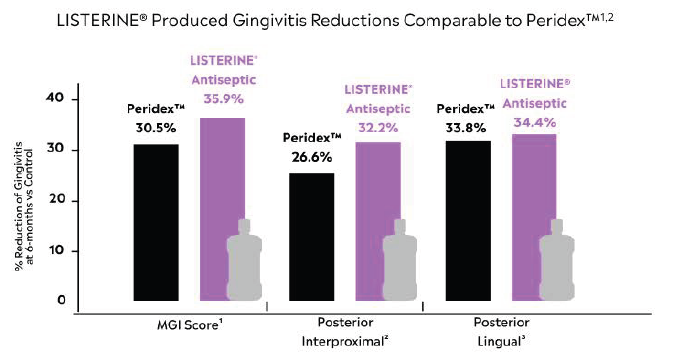
In fact, LISTERINE® Antiseptic is the only branded OTC rinse with gingivitis reduction comparable to prescription-strength Peridex™.16 To read about this study in more detail, click below:
LISTERINE® Antiseptic also compares favorably to other OTC antimicrobial mouthrinses, many of which contain CPC. Toothpaste can reduce the antimicrobial activity of CPC-containing mouthrinses, such as Crest® Pro-Health™, especially if used immediately after brushing. But LISTERINE® Antiseptic has no known interaction with toothpaste.2,3,16-18 Nor does it cause the significant extrinsic staining that is associated with both CPC and CHX.3-5
The LISTERINE® mouthrinse difference: Recognition by the ADA.
When recommending a mouthrinse to your patients, it is also important to consider the recognition of LISTERINE® Antiseptic by the American Dental Association (ADA) for efficacy and safety.19 In fact:

A LISTERINE® product for every patient need.
LISTERINE® offers a mouthrinse to suit the needs of virtually every type of patient. For example:
For patients 12 years and older who need to prevent plaque accumulation and gingivitis:
LISTERINE® COOL MINT® ANTISEPTIC MOUTHWASH
The classic LISTERINE® Antiseptic rinse, proven to kill the bacteria that cause plaque and gingivitis6
Dosing: Rinse with 20 mL for 30 seconds morning and night
LISTERINE® ULTRACLEAN® ANTISEPTIC MOUTHWASH
All the benefits of LISTERINE® Antiseptic, plus clinically proven to control calculus build-up
Dosing: Rinse with 20 mL for 30 seconds morning and night
For patients 6 years and older who need an alcohol-free option:
LISTERINE® COOL MINT® ZERO ALCOHOL MOUTHWASH
Alcohol-free formula with a less intense taste, proven to kill millions of bacteria that cause halitosis on contact20
Dosing: Rinse with 20 mL for 30 seconds morning and night
For a comparative look at the full line of products offered by LISTERINE®, sortable by patient need, click below:
What we've learned:
1. What is the key difference between therapeutic and cosmetic mouthwashes?
a.) Therapeutic mouthwashes can help reduce plaque
b.) Therapeutic mouthwashes can help reduce gingivitis
c.) Therapeutic mouthwashes can help reduce halitosis
d.) All of the above
2. What is the mechanism of action for the 4 Essential Oils formulation?
a.) Deeply penetrates plaque biofilm for superior bacteria-kill
b.) No interaction with toothpaste
c.) Lyses, kills, and inhibits the growth of both gram-positive and gram-negative bacteria
d.) All of the above
3. What are the dosing instructions for EO mouthwash?
a.) Rinse with 10 mL for 30 seconds morning and night
b.) Rinse with 10 mL for 60 seconds morning and night
c.) Rinse with 20 mL for 30 seconds morning and night
d.) Rinse with 20 mL for 60 seconds morning and night
Answers: 1. d; 2. d; 3. c.
*Lab study: mixed species biofilm growth 48 hours prior to 30-second treatment with mouthrinse or sterile water.
†The ADA Council on Scientific Affairs’ Acceptance of LISTERINE® Antiseptic is based on its finding that the product is effective in helping to prevent or reduce gingivitis and plaque above the gumline, when used as directed.
References:
1. American Dental Association. ADA Seal of Acceptance. Accessed May 12, 2016.
2. DePaola LG, Spolarich AE. Safety and efficacy of antimicrobial mouthrinses in clinical practice. J Dent Hygiene. 2007;81(special suppl):13-25.
3. Sheen S, Owens J, Addy M. The effect of toothpaste on the propensity of chlorhexidine and cetylpyridinium chloride to produce staining in vitro: a possible predictor of inactivation. J Clin Periodontal. 2001;28:46-51.
4. Addy M, Mahdavi SA, Loyn T. Dietary staining in vitro by mouthrinses as a comparative measure of antiseptic activity and predictor of staining in vivo. J Dent. 1995;23:95-99.
5. Addy M, Moran J. The formation of stain on acrylic surfaces by the interaction of cationic antiseptic mouthrinses and tea. J Biomed Mater Res. 1984;18:631-641.
6. Sharma N, Charles CH, Lynch MC, et al. Adjunctive benefit of an essential oil-containing mouthrinse in reducing plaque and gingivitis in patients who brush and floss regularly: a six-month study. J Am Dent Assoc. 2004;135(4):496-504.
7. Fine DH, Furgang D, Lieb R, Korik I, Vincent JW, Barnett ML. Effects of sublethal exposure to an antiseptic mouthrinse representative plaque bacteria. J Clin Periodontol. 1996;23(5):444-451.
8. Ricci-Nittel D, Fourre T. In vivo evaluation of antimicrobial activity of an essential-oil mouthrinse. Presented at: General Session of the Inter
national Association for Dental Research: March 16-19, 2011; San Diego, CA.
9. Fine DH, Letizia J, Mandel ID. The effect of rinsing with Listerine antiseptic on the properties of developing dental plaque. J Clin Periodontol. 1985;12:660-666.
10. Ross NM, Charles CH, Dills SS. Long-term effects of Listerine antiseptic on dental plaque and gingivitis. J Clin Dent. 1989;1(4):92-95.
11. Drake D, Villhauer AL. An in vitro comparative study determining bactericidal activity of stabilized chlorine dioxide and other oral rinses. J Clin Dent. 2011;22(1):1-5.
12. Andreana S, Nittel-Ricci D, Wu MM, Harper DS, Baxter KA. In vitro antimicrobial activity of vanilla mint Listerine® antiseptic mouthrinse. Presented at: IADR/AADR/CADR 85th General Session and Exhibition. March 2007.
13. Pan PH, Finnegan MB, Sturdivant L, Barnett ML. Comparative antimicrobial activity of an essential oil and an amine fluoride/stannous fluoride mouthrinse in vitro. J Clin Periodontol. 1999;26(7):474-476.
14. Fine DH, Furgang D, Barnett ML, et al. Effect of an essential oil-containing antiseptic mouthrinse on plaque and salivary Streptococcus mutans levels. J Clin Periodontol. 2000;27(3):157-161.
15. Data on file, Johnson & Johnson Consumer Inc.
16. Overholser CD, Meiller TF, DePaola LG, Minah GE, Niehaus C. Comparative effects of 2 chemotherapeutic mouthrinses on the development of supragingival dental plaque and gingivitis. J Clin Periodontol. 1990;17:575-579.
17. Sheen S, Addy M. An in vitro evaluation of the availability of cetylpyridinium chloride and chlorhexidine in some commercially available mouthrinse products. Br Dent J. 2003;194:207-210.
18. Sheen S, Eisenburger M, Addy M. Effect of toothpaste on the plaque inhibitory properties of a cetylpyridinium chloride mouthrinse. J Clin Periodontol. 2003;30:255-260.
19. American Dental Association. ADA seal product report: Listerine® antiseptic. ADA Web site. https://www.ada.org/resources/research/science-and-research-institute. Accessed May 9, 2014.
20. Ilg D, McGuire JA, Mordas CJ, Queiroz D, Fourre T, Santos SL. In vitro efficacy of an alcohol-free essential oil containing mouthrinse. J Dent Hyg. 2012;86(1):50-51.