§In smokers, by reducing then stopping Nicorette® completely after quitting smoking
*Abstinence rates: Combination Therapy (nicotine patch + active gum): 27.5% vs nicotine patch + placebo gum: 15.3%:
p=0.01, at 24 weeks47
You can help your patients today
8/10 smokers and 6/10 vapers want to reduce or quit4
96% smokers will fail to quit if they rely on willpower alone5
Increased chance of quiting smoking with Healthcare Practitioners support6,7
Explaining alternative tobacco and
nicotine products
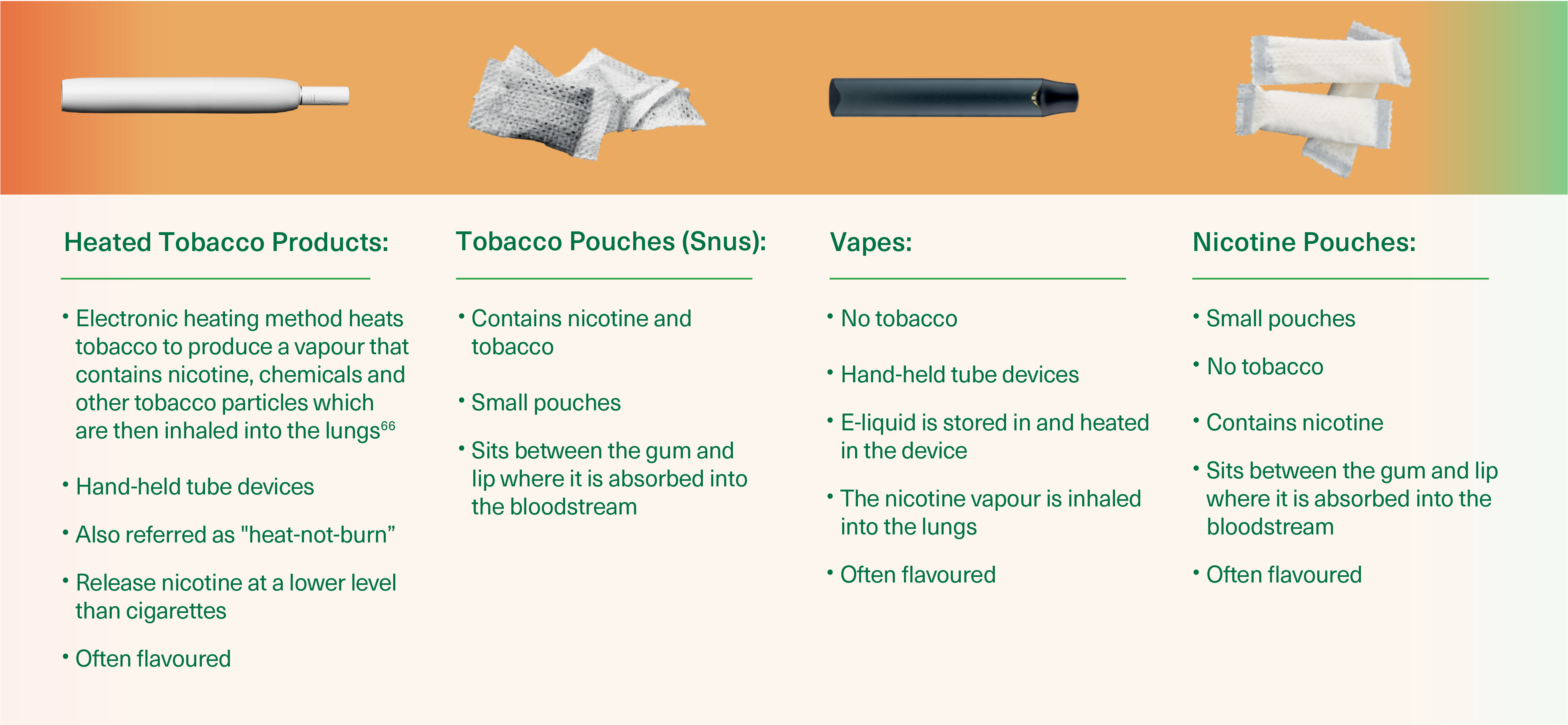
They are often perceived and used as a possible route to quitting smoking and can be a step in the right direction, however many smokers who start using alternatives to try to quit smoking will instead be drown into a cycle of continued nicotine addiction.
They are safer than smoking cigarettes. But safer does not necessarily mean safe. If your patients do not smoke, they shouldn't use alternative tabaco or nicotine products.
Scale of health risk8,9

*Cigarettes & alternative tobacco and nicotine products mostly owned and manufactured by tobacco companies22,23,24.25
Alternative tobacco and nicotine products:
What you need to know
Whilst alternative tobacco and nicotine products could be a step in the right direction, most have not been specifically developed to aid quitting:
Average weekly nicotine consumption amongst vapers is 2X that of an average smoker34-36
70% of alternative tobacco and nicotine product users still smoke37

Alternative tobacco and nicotine products are not licensed medicines
No clear dosing regimen and inconsistent dose delivery
No tapering guidance
Often flavoured for purposes of enjoyment
May contain harmful ingredients
Long-term health risks largely unknown

Alternative tobacco and nicotine products in adolescents
Studies∆∆, show that nicotine binds to more receptors in the brains of teenagers than in adults. This results in a greater release of dopamine, making the experience of nicotine more rewarding for teenagers than for adults. Nicotine also affects the systems that release other neurochemicals like noradrenaline and serotonin, leading to different experiences in mood, reward, and decision-making for teenagers compared to adults38-40
Alternative tobacco and nicotine products are often designed to appeal to adolescents through lifestyle branding and flavours
Adolescent brains are more susceptible to impulsive decision making40
Younger age groups are more likely to continue usage (obtaining greater pleasure from nicotine use than older age groups)
Nicotine has been associated with decreased academic performance and higher drop out rates in youth41
If your patients do not smoke, they shouldn't use alternative tobacco or nicotine products
Nicorette® is licensed to help smokers or vapers to quit and can be used in children from the age of12

Unlike alternative tobacco and nicotine
products, NRT:
NRTs are licensed medicines:1-3
Licensed, with a good safety profile and a defined dosing schedule for cessation
Clinically indicated to relieve and/or prevent cravings and nicotine withdrawal symptoms associated with tobacco dependence
Pharmaceutical grade nicotine in measured doses29
NRT is designed and acknowledged to help patients become smoke and nicotine free:1-3 ** §§
Endpoint aim of complete cessation
Many clinical guidelines globally recommend NRT as a first-line treatment for smoking cessation42-44
On the WHO list of essential medicines43
**Abstinence rates: Combination Therapy (nicotine patch + active gum): 27.5% vs nicotine patch + placebo gum: 15.3% p=0.01, at 24 weeks.47
§In smokers by reducing then stopping Nicorette® completely after quitting smoking
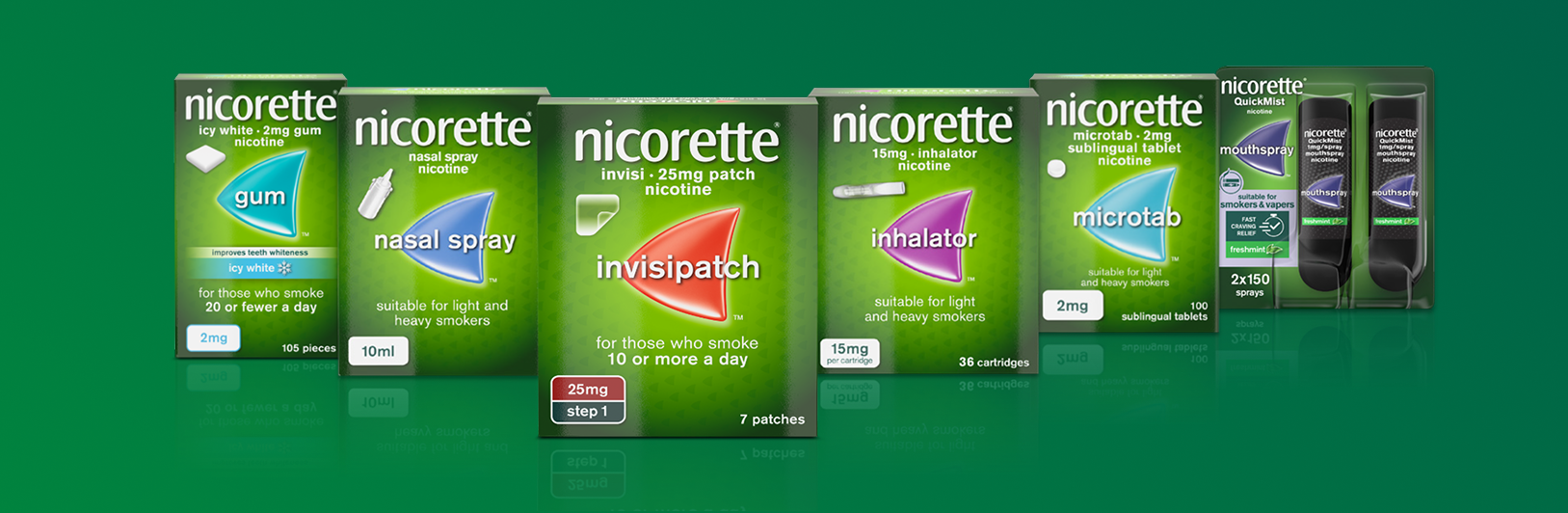
NRT is available in formats that can be utilised in combination therapy:Nicorette® Invisipatch provides long-lasting craving relief over 16 hours3 (specifically designed to be removed at night45)

Nicorette® Quickmist and SmartTrack mouth spray, gum, lozenge, nasal spray, inhalator or microtab for on-demand craving relief46
Delivering both long-lasting and faster-acting craving relief to provide sustained levels of background nicotine and relief from breakthrough cravings

How does NRT work?
Nicotine that is inhaled from tobacco smoke or alternative tobacco and nicotine products enters the blood circulatory system and rapidly crosses the blood-brain barrier48
By binding to nicotinic acetylcholine receptors (nAChRs) in the brain and opening channels for neurotransmitters, nicotine stimulates the release of neurotransmitters, including dopamine and glutamate49
Increased nicotine use leads to more of these receptors, increasing craving and dependency leading to even higher nicotine use50
The use of alternative tobacco and nicotine products is likely to contribute to this increasing cycle of consumption, with sustained or increased nicotine use as the result
Nicorette® disrupts the cycle of higher nicotine use by providing therapeutic doses of nicotine that will reduce cravings in a dosing regimen that can be tapered down.51,52 With the brain down regulating the number of nAChRs, cravings are further reduced leading to increased chances of successfully and completely quitting nicotine53,54
Nicorette® Quickmist spray is proven to start to relieve the urge to smoke within 30 seconds, and is indicated for smoking cessation1
Nicorette® helps your patients enjoy freedom smoking and vaping.
Help patients quit smoking with Nicorette®

Nicorette® products provide effective craving relief in smokers,55-57, backed by 45+ years of real-world experience and over 240+ clinical studies58,59
Nicorette® Combination Therapy is a clinically proven quit smoking method that gives patients control over46,60:
Background withdrawal, with Nicorette® patch
Breakthrough cravings with a fast acting format:
Nicorette Quickmist can start to relieve cravings in 30 seconds61
NRT including Combination Therapy support the relief of 7 nicotine withdrawal symptoms: the relief of urges to smoke (cravings), depressed mood, insomnia, irritability, frustration or anger, anxiety, difficulty in concentrating, restlessness, and increased appetite or weight gain1-3
Nicorette® offers a broad range of products which can be tailored to different needs
Even less than 3 minutes can save a life

‘Have you smoked in the last 30 days?’
‘Would you like to stop smoking today?’
As HCP
You can save a life in less than 3 min. Ask all your patients
For Patient
Quitting smoking is one of the best things patients can do for their health10
Smoking is the No.1 preventable cause of death globally62

Empathise and advise that the best way to quit smoking is with non-smoking aids, like NRT and your/a specialist support (VBA*)
Help them set their expectations on their quit journey (learnings from previous attempts can be used to help this one)63
As HCP
85% of patients expect and want their physician to bring up the topic of smoking cessation64
For Patient
The combination of non-smoking aids, like NRT and your behavioural support can boost their chances of quitting by 3 to 5 times6,7

Recommend stop smoking aids like NRT combination therapy or prescription medicines and provide your ongoing support
Set the quit date with your patient
Offer a follow-up appointment with you or a specialistAs HCP
You can support your patient and build confidence, for example, by referring the patient to additional smoking cessation resources
For patient
There are health benefits related to quitting
* VBA = Very Brief Advice
No other over-the-counter medicine is more effective than Nicorette® Combination Therapy at helping your patients quit smoking for good65
Nicorette® is available to purchase in stores or pharmacy's

Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/
Adverse events should also be reported to McNeil Products Limited on freephone 0808 238 9999.
References
1. Nicorette® QuickMist 1mg/spray mouthspray. Summary of Product Characteristics.
2. Nicorette® 2mg gum. Summary of Product Characteristics.
3. Nicorette® Invisi25mg patch. Summary of Product Characteristics.
4. Incite 2024. Kenvue Global CEPs Usage and Attitude Report.
5. Dono J, et al. Lancet Reg Health West Pac. 2021;19:100342.
6. West, R. and Papadakis, S. (2019) Stop smoking services: increased chances of quitting. London; National Centre for Smoking Cessation and Training.
7. Sutherland G. Heart. 2003;89 Suppl 2(Suppl 2):ii25-ii37.
8. Royal College of Physicians UK, E-Cigarettes and Harm Reduction – an evidence review, April 2024. https://www.rcp.ac.uk/media/n5skyz1t/e-cigarettes-and-harm-reduction_full-report_ updated_0.pdf
9. Kalkhoran S, Benowitz NL, Rigotti NA. Prevention and Treatment of Tobacco Use: JACC Health Promot ion Series. J Am Coll Cardiol. 2018 Aug 28;72(9):1030-1045. doi: 10.1016/j. jacc.2018.06.036. PMID: 30139432; PMCID: PMC6261256.
10. U.S. Department of Health and Human Services. Smoking Cessation. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2020.
11. Cohen et al. Am J Pub Health 1993; 83(9): 1277-1283.
12. Reibel Med Princ Pract 2003; 12(suppl 1): 22-32, Johnson J Periodonl 2004; 75(2): 196-209.
13. Dietrich et al. J Dent ResI 2007; 86(4): 373-377, Albandar et al. J Periodonl 2000; 71(12): 1874-1881.
14. Kurata & Nogawa J Clin Gastroenterol 1997; 24(1): 2-17.
15. Znyk M, et al. Int J Environ Res Public Health. 2021;18(12):6651.
16. Pataka A, et al. Medicina (Kaunas). 2020;56(6):292.
17. Nabavizadeh P, et al. Tob Control. 2018;27(Suppl 1):s13–s19.
18. Miyashita L, et al. J Allergy Clin Immunol. 2018;141:AB28.
19. Byhamre ML, et al. Int J Epidemiol. 2021;49(6):2041-2050.
20. McConnell R, et al. Am J Respir Crit Care Med. 2017;195(8):1043–1049.
21. Schweitzer RJ, et al. Prev Med. 2017;105:226–231.
22. Choi K, BernatD. Am J Prev Med. 2016;51(4):446–453.
23. Callahan-Lyon P. Tob Control. 2014;23 Suppl2(Suppl2):ii36–ii40.
24. Sharma A, et al. Int J Cardiol. 2023;371:65–70.
25. Bayly JE, et al. Chest. 2019;155(1):88–93.
26. Iacob AM, et al. Medicina (Kaunas). 2024;60(3):365.
27. Rusiecka AW et al. J Pre-Clin and Clin Res 2024;18(3):224-230.
28. Ye D, Rahman I. Int J Dent. 2023;2023:9437475.
29. European Pharmacopeia (Ph. Eur.). 11th edition. Strasbourg, France: EDQM Council of Europe. Nicotine 1452 (01/2009:1452) respectively Nicotine Resinate(01/2015:1792).
30. Euromonitor International, Passport, Vape/Ecigarettebrands and manufacturers market data, 2018 –2023.
31. Euromonitor International, Passport, Nicotine Pouches manufacturers market data, 2018–2023.
32. Cigarette Companies Investing in Snus, Tobacco Tactics, updated 20 March 2024, accessed 23 May 2024. https://tobaccotactics.org/article/cigarette-companies-investing-in-snus/.
33. Heated Tobacco Products, Tobacco Tactics, updated 08 April 2024. https://tobaccotactics.org/article/heated-tobacco-products/ (accessed Apr 2025).
34. Data on file. Basis, 2023, Vaping Truths Diary Study, n=100.
35. Jewell, T. 18 November 2019. How Much Nicotine Is in a Cigarette, Cigar, and Other Tobacco Products? Available at: https://www.healthline.com/health/how-much-nicotine-is-in-a-cigarette. Accessed July 2024. 36. NHS Digital 2021. Health Survey for England 2021. Adults' health-related behaviours. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2021/adults-health-related-behaviours. Accessed July 2024.
37. Data on file. Incite. May 2023, UK Nicotine Landscape. Main study (discordant nicotine users).
38. Leslie, F.M., 2020. Unique, long-term effects of nicotine on adolescent brain. Pharmacology Biochemistry and Behavior, 197, p.173010.
39. Yuan, M., Cross, S.J., Loughlin, S.E. and Leslie, F.M., 2015. Nicotine and the adolescent brain. The Journal of physiology, 593(16), pp.3397-3412.
40. Lydon, D.M., Wilson, S.J., Child, A. and Geier, C.F., 2014. Adolescent brain maturation and smoking: what we know and where we’re headed. Neuroscience & Biobehavioral Reviews, 45, pp.323-342.
41. Gage SH, et al. Psychol Med. 2022;52(8):1578–1586.
42. Canada Alberta Health Services Tobacco, Vaping and Cannabis Program – June 2021.
43. World Health Organisation, Model List of Essential Medicines, 23rd list 2023, WHO Model List of Essential Medicines – 23rd list, 2023.
44. NICE guidance. Tobacco: preventing uptake, promoting quitting and treating dependence [NG208] Nov 2021.
45. Data on file.
46. Nicorette® UK. Nicorette Dual Support. Available at: https://academy-plus.co.uk/page/nonprescribercombinationtherapy (accessed Apr 2025).
47. Kornitzer M, et al. Prev Med. 1995;24(1):41–47.
48. Tega Y, et al. Biol Pharm Bull. 2018;41(9):1330–1336.
49. Wittenberg RE, et al. Neuropharmacology. 2020;177:108256.
50. Benowitz NL. Annu Rev Pharmacol Toxicol. 2009;49:57–71.
51. Sandhu A, Hosseini SA, Saadabadi A. Nicotine Replacement Therapy. [Updated 2023 Nov 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan.
52. Sweeney CT, et al. CNS Drugs. 2001;15(6):453–67.
53. Benowitz NL. Clin Pharmacol Ther. 2008;83(4):531–541.
54. Molyneux A. BMJ. 2004;328(7437):454–456.
55. Hansson A, et al. BMJ Open. 2012;2(5):e001618.
56. Shiffman S, et al. J Consult Clin Psychol. 1996;64(2):366–79.
57. Tønnesen P, et al. Eur Respir J. 2012;40(3):548–54.
58. Fernö, O. Addiction;1994;89,1215–1226.
59. Kenvue internal data.
60 Theodoulou A, et al. Cochrane Database Syst Rev. 2023;6(6):CD013308.
61. Data on file; QuickMist vaping study, 2022.
62. https://www.who.int/news-room/fact-sheets/detail/tobacco.
63. NCSCT standard treatment programme 2019. 5. Slama KJ, et al. Family Practice 1989;6(3):203-209.
64. Slama KJ, et al. Family Practice 1989;6(3):203-209.
65. Lindson N, et al. Cochrane Database Syst Rev. 2019;4(4):CD013308.
66. Cancer Research UK. Smokeless tobacco: 5 common questions about ‘heat not burn’ products answered; https://news.cancerresearchuk.org/2019/02/01/smokeless-tobacco-5-common-questions-about-heat-not-burn-products-answered/ (accessed Apr 2025).
67. European Respiratory Society. ERS position Paper on Heated Tobacco Products; The organization: Lausanne, Switzerland, 2018 ; Available online.; https://www.ersnet.org/news-and-features/news/ers-position-paper-on-heated-tobacco-products/ (accessed Jan 2025)
