Discontinuation Due to Somnolence in Adult Clinical Trials1
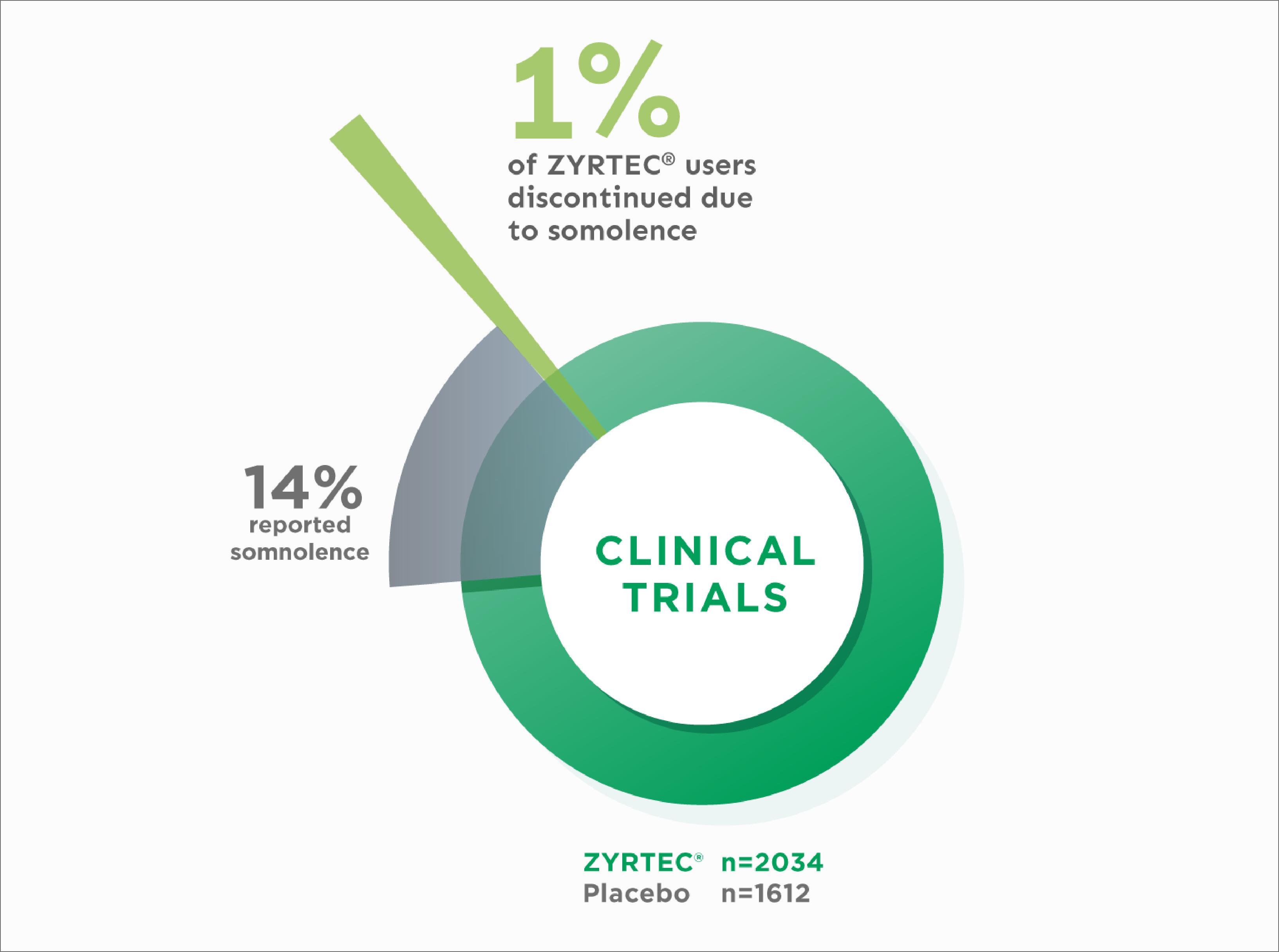
Discontinuation due to adverse events in clinical trials was not significantly different between ZYRTEC® and placebo (2.9% vs 2.4%, respectively)1
14% reported somnolence with ZYRTEC® vs 6% with placebo
Somnolence rates 1
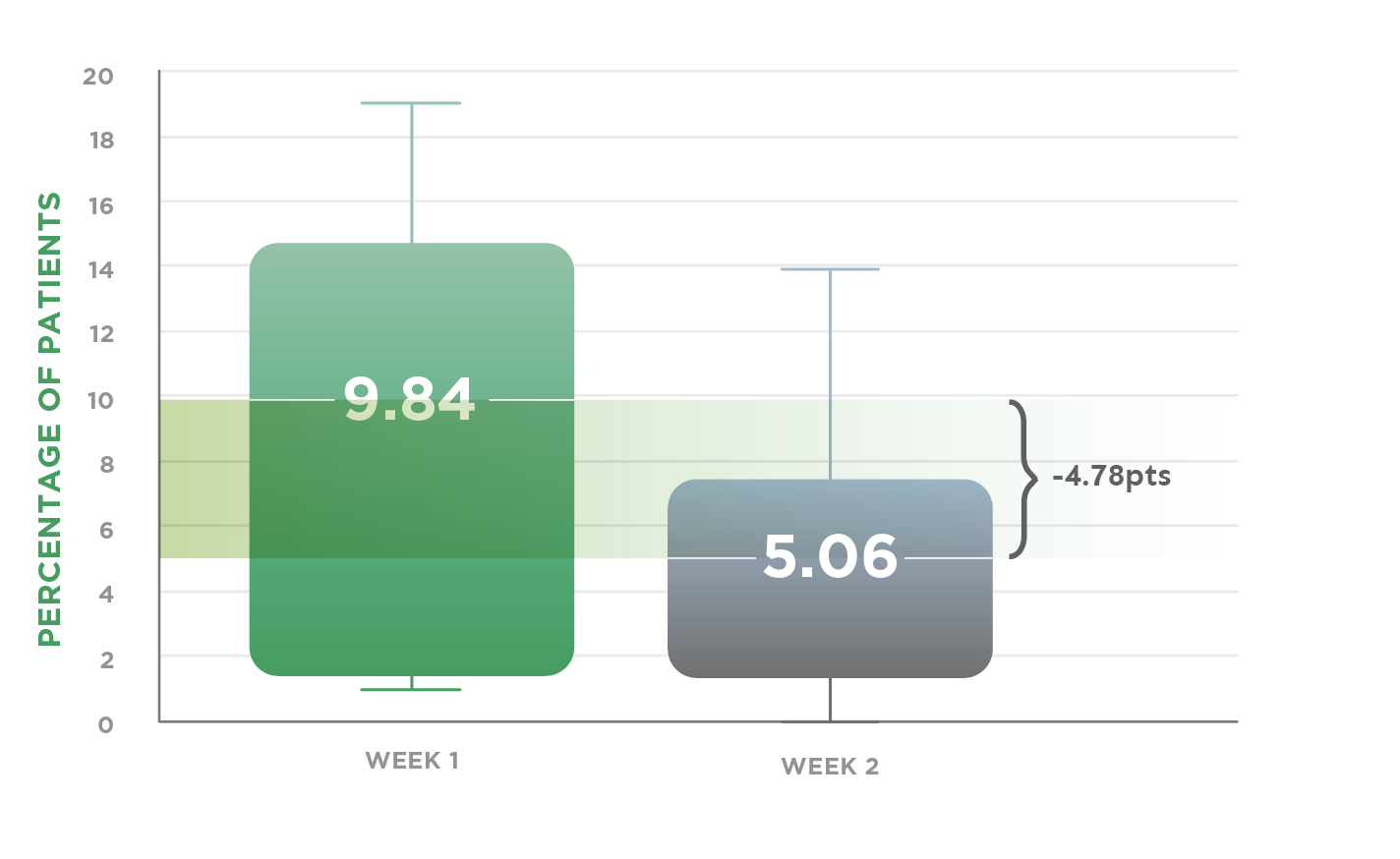
Of the patients reporting somnolence, the majority did so in the first week, with the rates decreasing at week 21*†
Studies show ZYRTEC® is also generally well tolerated by children.1
See pediatric data
*Retrospective analyses of data from 8 randomized, placebo-controlled allergic rhinitis studies SAR and PAR, at least 2-week long.
†Distribution of percent (%) of safety-evaluated patients who reported somnolence after receiving a single daily dose of cetirizine 10 mg, for at least 2 weeks. Somnolence rates are shown for weeks 1 and 2. Horizontal line inside the chart box is representative of the median.
Reference:
1. Data on file, Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division.
