Studies used the Rhinoconjunctivitis Quality of Life
Questionnaire (RQLQ), a validated, allergy-specific measurement of overall QOL. Two of the studies used the 28-question version of the RQLQ, which measures7 domains4§:
Activity limitation
Sleep problems
Nose symptoms
Eye symptoms
Non–hay fever symptoms
Practical problems
Emotional function
In both perennial and seasonal allergic rhinitis, ZYRTEC® significantly improved adult patients’ QOL vs placebo1-3||¶
A study in patients with perennial allergic rhinitis (PAR) used a 24-question version of the RQLQ, which measure 6 allergy-related domains: activity limitation, sleep problems, nose symptoms, non–hay fever symptoms, practical problems, and emotional function.4§
Improvement in Quality of Life in Adults With PAR2||
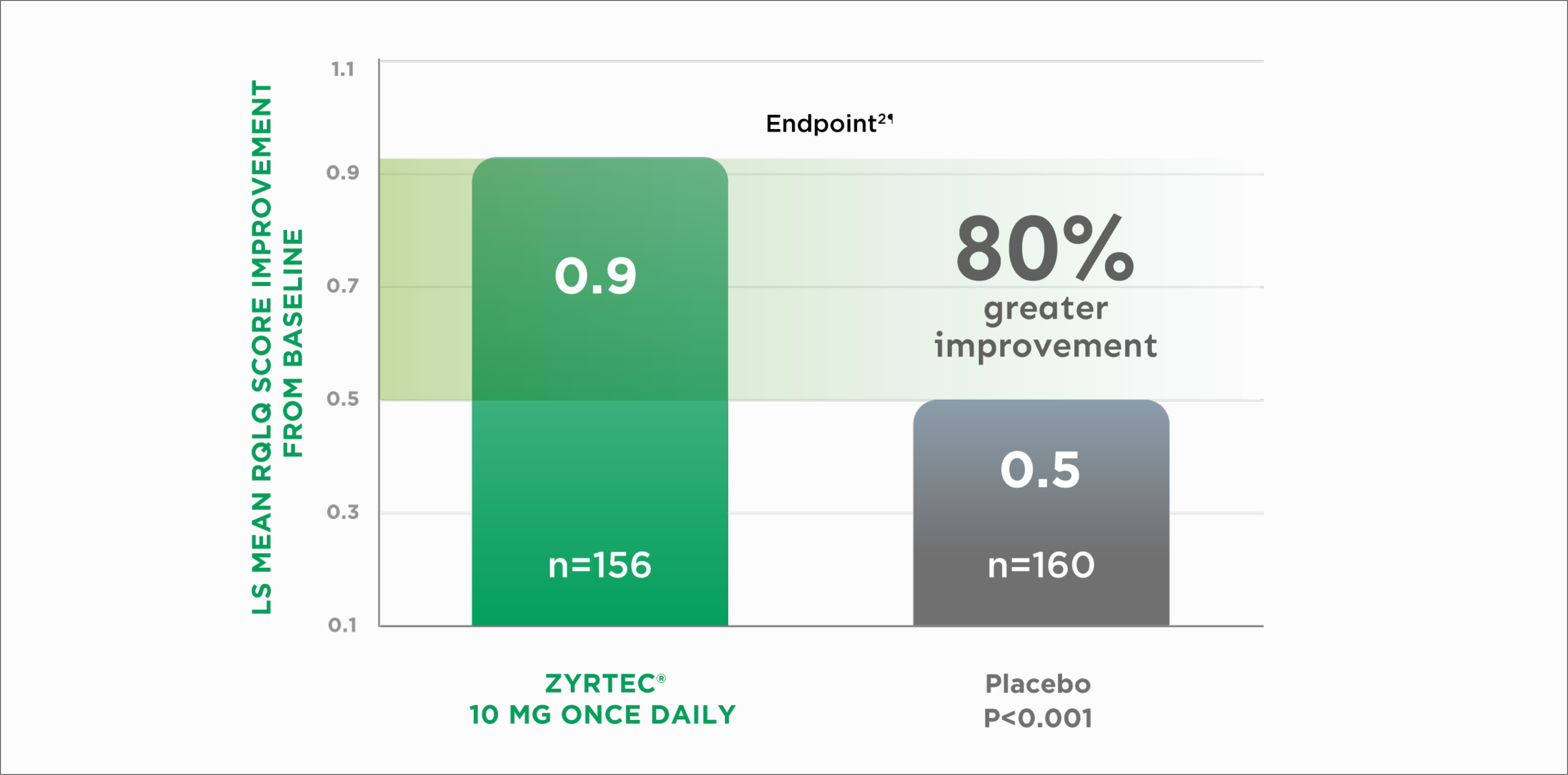
LS=Least squares
RQLQ=Rhinoconjunctivitis Quality of Life Questionnaire
Studies in patients with seasonal allergic rhinitis (SAR) used the 28-question version of the RQLQ that measured 7 allergy-related domains.
Improvement in Quality of Life in Adults With SAR2¶
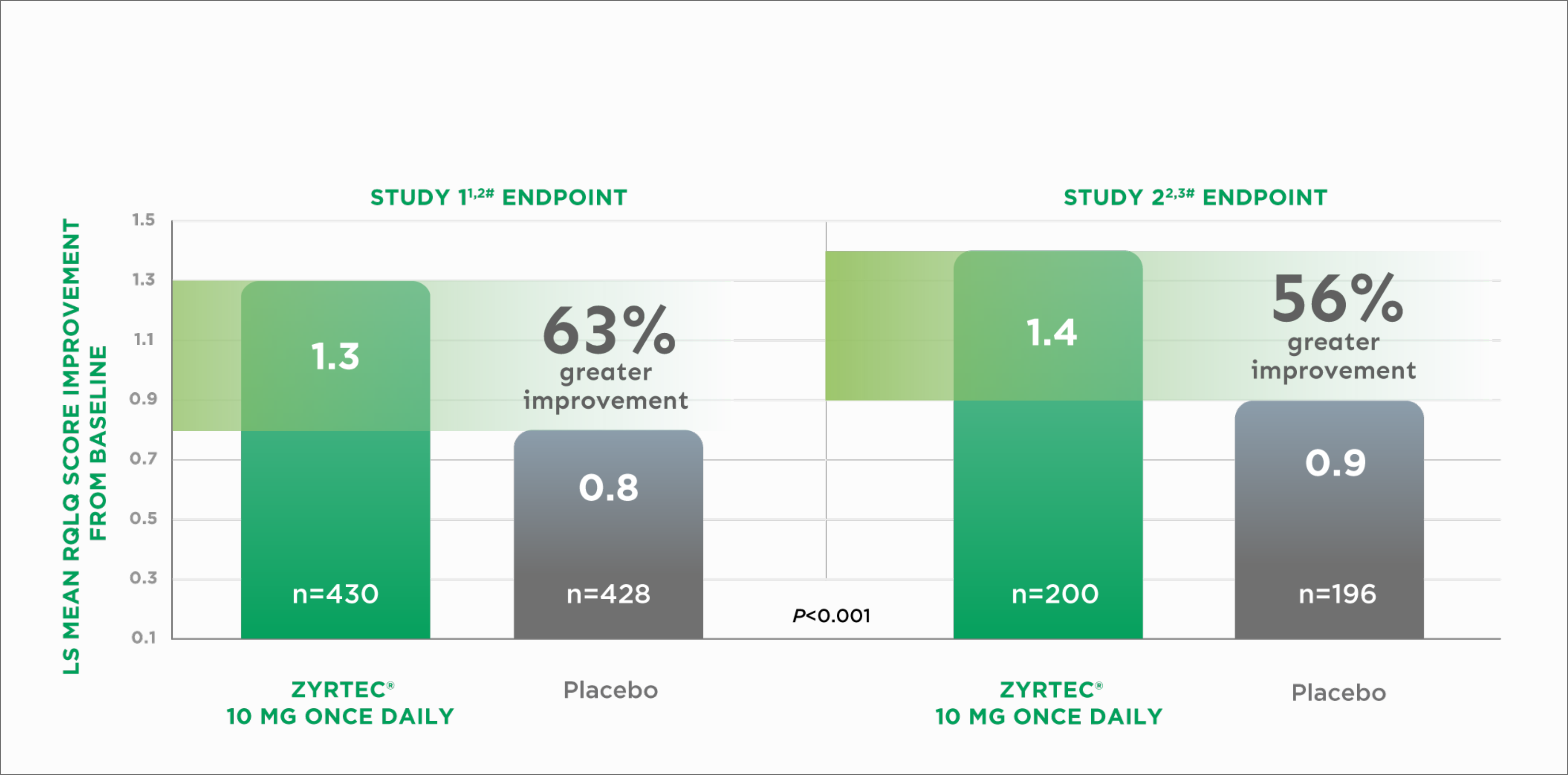
LS=Least squares
RQLQ=Rhinoconjunctivitis Quality of Life Questionnaire
Work-Related Data
This study assessed the impact of allergy treatment on self-reported activity impairment using the Work Productivity and Activity Impairment-Allergy Specific (WPAI-AS) questionnaire, which measures performance based on‡:
impairment at work (limitations in the amount or kind of work done)
activity impairment (limitations in usual activities, such as housework or shopping)
Reduction in Work Impairment in Adults With Seasonal Allergic Rhinitis1*‡
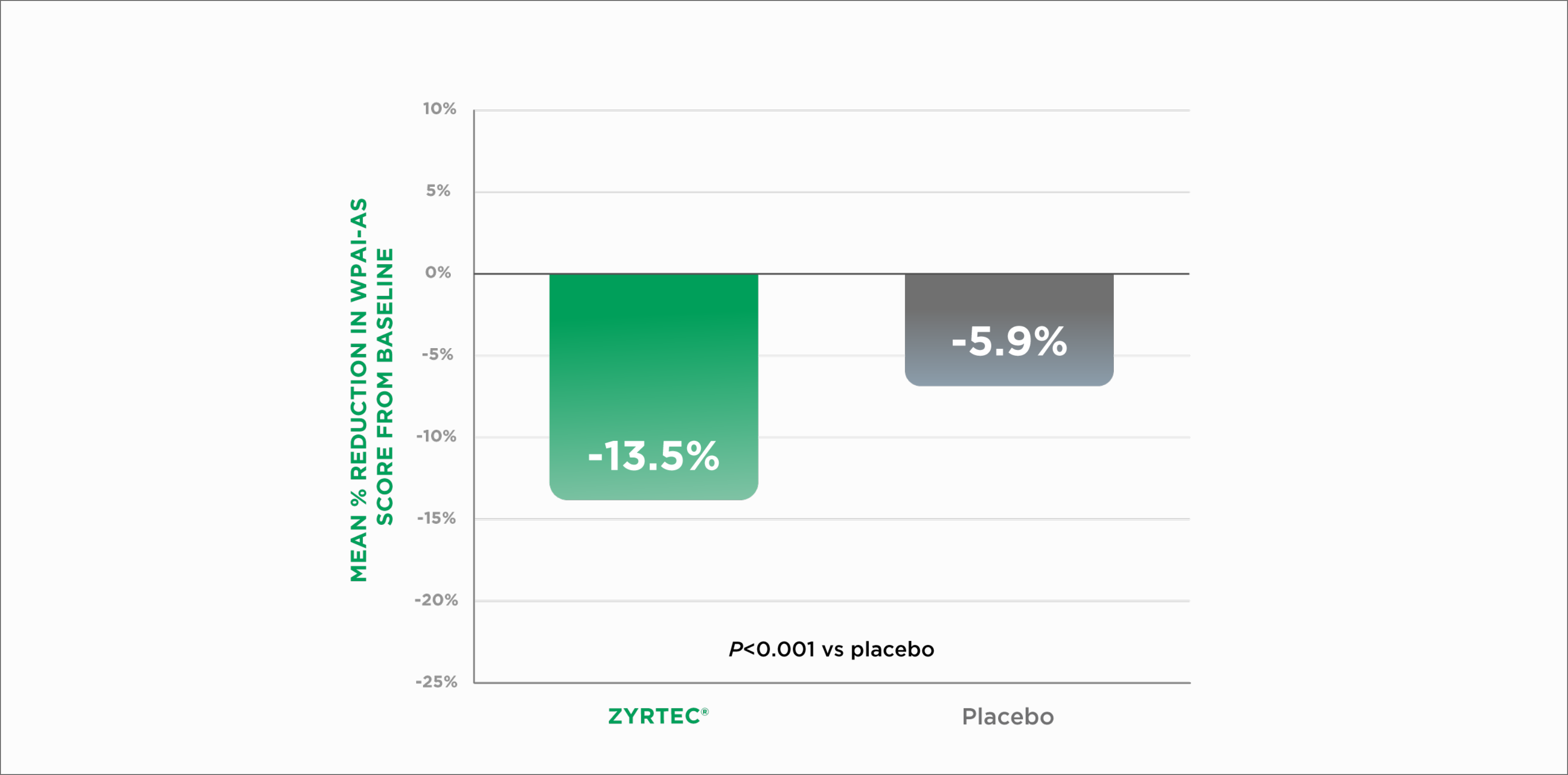
WPAI-AS=Work Productivity and Activity Impairment-Allergy Specific
*Randomized, placebo-controlled, 2-week study of adult patients with seasonal allergic rhinitis (n=431 for each treatment group).
†Patient disease-specific quality of life was assessed using the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ), which measures 7 domains: activity limitation, sleep problems, nose symptoms, eye symptoms, non–hay fever symptoms, practical problems, and emotional function.
‡Impact on self-reported activity impairment was assessed using the Work Productivity and Activity Impairment-Allergy Specific (WPAI-AS) questionnaire, which measures performance based on impairment at work (limitations in the amount or kind of work done, work accomplished, or work done as carefully as usual) and activity impairment (limitations in usual activities, such as work around the house, shopping, child care, exercising, etc).
§Adult RQLQ assessed domains on a 7-point (0-6) rating scale.
||Multicenter, randomized, double-blind, placebo-controlled study including 316 adult PAR patients. Primary study endpoint measured changes in overall RQLQ score from baseline.
¶Two multicenter, randomized, double-blind, placebo-controlled studies including 1254 adult SAR patients. Primary study endpoint measured changes in overall RQLQ score from baseline.
#Endpoint=last post-baseline observation.
Reference:
1. Murray JJ, Nathan RA, Bronsky EA, Olufade AO, Chapman D, Kramer B. Comprehensive evaluation of cetirizine in the management of seasonal allergic rhinitis: impact on symptoms, quality of life, productivity, and activity impairment. Allergy Asthma Proc. 2002;23(6):392-398.
2. Data on file, Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division.
3. Noonan MJ, Raphael GD, Nayak A, et al. The health-related quality of life effects of once-daily cetirizine HCI in patients with seasonal allergic rhinitis: a randomized double-blind, placebo-controlled trial. Clin Exp Allergy. 2003;3(3):351-358.
4. Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy. 1991;21(1):77-83.
