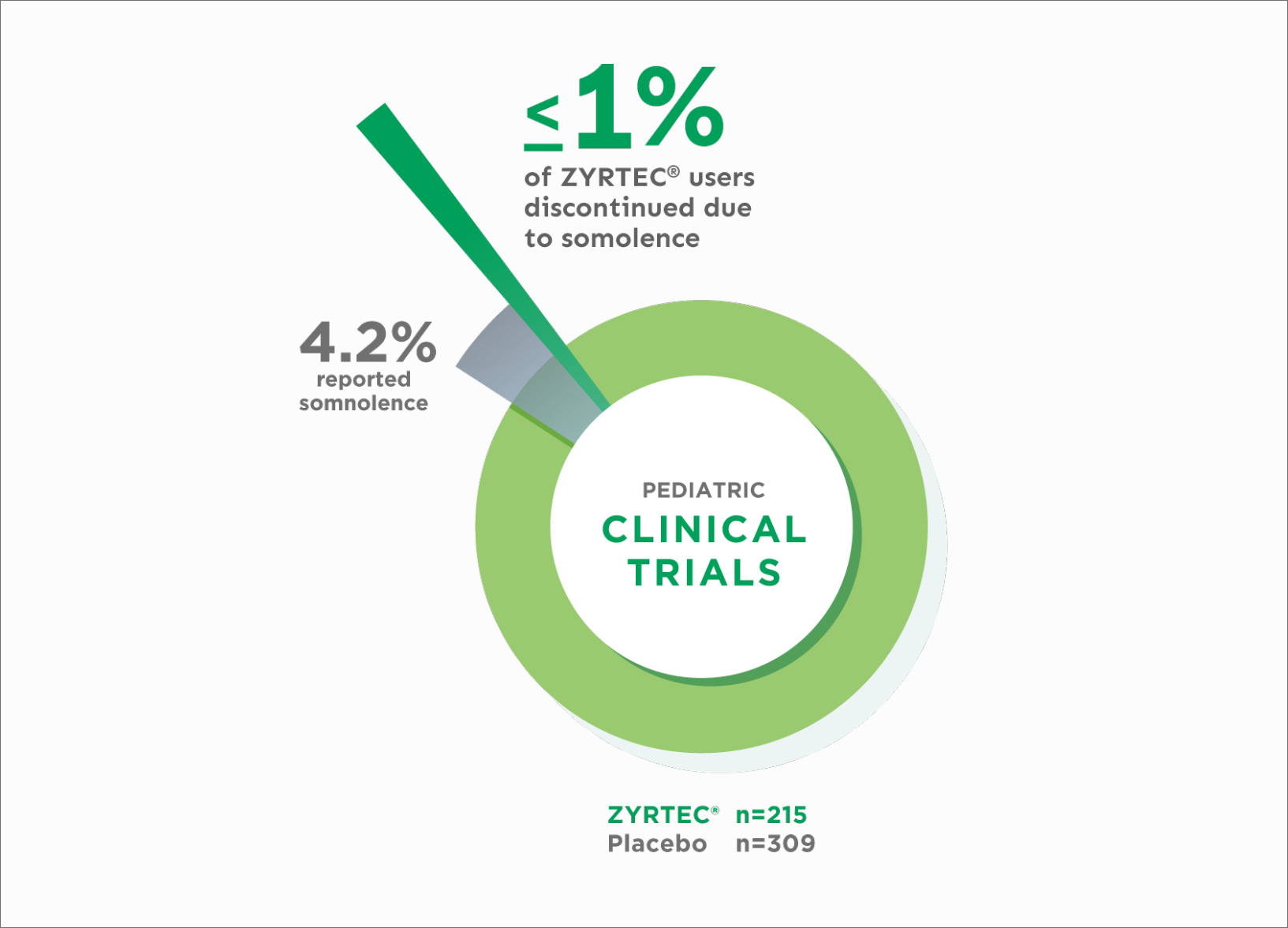
In studies of children ages 6 to 11 years:
4.2% of patients taking ZYRTEC® 10 mg reported somnolence (n=215) vs 1.3% with placebo (n=309)1
≤1% of ZYRTEC® patients discontinued due to somnolence1
Tolerability: Discontinuation Due to Somnolence in Clinical Trials of Children Ages 6 to 111
Reference:
1. Data on file, Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division.
