Key Words
Retinol, clinical study Antiaging efficacy, skin Gene expression
Abstract
The antiaging efficacy of retinol (ROL) has been explored mainly clinically in photoprotected skin sites and for high doses of ROL (0.4–1.6%). The objective of the study was to demonstrate the antiaging action of a low and tolerable dose of ROL (0.1%) ex vivo by measuring the expression of cellular retinoic-acid-binding protein II (CRABP2) and heparin-binding epidermal growth factor (HBEGF) by a histological evaluation of the epidermis and in vivo by assessing major aging signs and performing three-dimensional profilometry and digital imaging during a 9-month doubleblind placebo-controlled study involving 48 volunteers. Finally, epidermal cell proliferation was evaluated using tryptophan fluorescence spectroscopy. Our results demonstrate that 0.1% ROL induced CRABP2 and HBEGF gene expression and increased keratinocyte proliferation and epidermal thickness. In human volunteers, topical application of a ROL-containing product improved all major aging signs assessed in our study (wrinkles under the eyes, fine lines and tone evenness). Moreover, tryptophan fluorescence increased in the active-agent-treated group and not in the placebo-treated group, indicating that cell proliferation was accelerated in vivo. These data demonstrate that a product containing a low dose (0.1%) of ROL promotes keratinocyte proliferation ex vivo and in vivo, induces epidermal thickening ex vivo and alleviates skin aging signs, without any significant adverse reaction. Copyright © 2009 S. Karger AG, Basel
Introduction
Clinically, skin aging is associated with a variety of signs such as wrinkles, uneven pigmentation, skin roughness and laxity. These clinical features are consecutive to structural and metabolic changes that occur during the passage of time per se (chronological aging) or due to the effect of external factors such as repeated exposure of skin sites to solar ultraviolet (UV) radiation (photoaging). Among these changes there is dermal thinning, which is consecutive to a decrease in fibroblast number as well as collagen synthesis and an increase in UV-induced collagen degradation by matrix metalloproteinases. In addition, as a constant hallmark of skin aging and photoaging there is epidermal thinning, which is triggered by a decrease in keratinocyte turnover rate [1] . Beneficial effects of all- trans -retinoic acid (ATRA) on skin photoaging are now well documented. For instance, it has been demonstrated that ATRA at 0.025 or 0.1% improves skin photoaging signs [2, 3] , and this clinical efficacy was mainly attributed to the effect of ATRA on collagen metabolism. In fact, ATRA has been shown to stimulate the synthesis of collagen that ultimately accumulates in the upper part of the papillary dermis [3] . Moreover, ATRA downregulates UV-induced matrix metalloproteinase 1 and 9 expression [4, 5] , thereby replenishing collagen levels. Although ATRA is recognized as an effective therapy for the treatment of photoaged skin through its regulatory effect on collagen metabolism, it has been suggested that retinol (ROL), also known as vitamin A, which shows a better irritation profile, may also alleviate some major signs of photoaging. Indeed, ROL at 1% exerts retinoic-acid-like effects such as stimulation of collagen synthesis, reduced matrix metalloproteinase levels and induction of fibroblast outgrowth [6] . However, most recent studies have only focused on the clinical benefits of ROL in photoprotected skin sites such as the upper arm, but not in photoexposed sites. For instance, it has been shown that long-term application of ROL at 0.4% to the upper arms reduces fine lines and provokes an accumulation of collagen in the papillary dermis [7] . Moreover, a vast majority of studies describe the effect of ROL at high doses ranging from 0.4 to 1.6% [6, 8] , doses at which it may produce unwanted side effects such as skin dryness, irritation and itching [9] . Lastly, at high concentrations, ROL is expected to exert dermal effects, but at lower, more tolerable doses, ROL may primarily exert biological activities in the epidermis. Although it has been previously demonstrated by histochemistry that 0.1% ROL increases epidermal thickness when applied in a propylene glycol/ethanol mixture under an occlusive patch for 4 days [8] , to our knowledge, the effect of ROL on epidermal cell proliferation has not been investigated in vivo in face skin, after a normal longterm usage of a skin care product containing ROL. Actually, epidermal thickness and keratinocyte proliferation are usually assessed by immunohistochemistry procedures on skin biopsies, and this technique cannot be easily performed on face skin. Recently, in vivo noninvasive methods that can objectively measure parameters related to skin biology and that complement the more subjective clinical evaluation have been developed [10] . In the case of skin aging, in vivo fluorescence spectroscopy provides an important marker that has been shown to relate epidermal thickness and epidermal cell proliferation rate in mice [11] and in humans [12–14] . Up to now, fluorescence spectroscopy has not been used to measure epidermal proliferation induced by ROL. In the present study, we explored the antiaging action of a low concentration of ROL (0.1%) on human skin. First, we tested the hypothesis that 0.1% ROL can induce increases in keratinocyte metabolism and proliferation ex vivo (human skin explant culture). Second, we attempted to document the ROL-induced antiaging effects in vivo using noninvasive methods including tryptophan fluorescence and clinical evaluation.
Materials and Methods
Products
In all experiments, the products tested were: (a) a cosmetic formulation containing 0.1% ROL, designated as ‘active’, and (b) the same formulation without ROL, designated as ‘placebo’.
Ex vivo Study
An ex vivo study was performed using human skin explants. Abdominal skin samples were obtained from normal human adults undergoing abdominoplasty surgery. Informed consent was obtained from each patient, and all experimentation steps were approved by an internal review board. Subcutaneous fat was carefully removed, and skin biopsies of 0.93 cm 2 were prepared under sterile conditions and placed in keratinocyte growth medium (Lonza, Saint-Beauzire, France) under a 5% CO 2 humidified atmosphere overnight.
Analysis of Gene Expression by Real-Time Quantitative Polymerase Chain Reaction. Skin explants were treated by topical application of a formulation containing 0.1% ROL and were incubated for 24 and 48 h. At the end of incubation, the epidermis was separated from the dermis by heat treatment, homogenized in Trireagent (Sigma, Saint-Quentin-Fallavier, France) and stored at –80 ° C until further RNA extraction. RNA from skin explants was extracted by the acid guanidium-thiocyanate-phenol-chloroform method using Trireagent. The concentration of total RNA was determined by measuring the optical density at 260 nm. The purity of the RNAs was assessed by measuring A260/A230 and A260/A280 ratios.
One microgram of total RNA was reverse transcribed to generate first-strand cDNA using the Improm-II Reverse Transcription system (Promega, Charbonnières, France) with random hexamers as suggested in the manufacturer instructions. As controls, parallel reactions were run in the absence of reverse transcriptase or in the absence of input RNA to assess any genomic DNA contamination.
Real-time quantitative polymerase chain reaction (QPCR) amplification was carried out using the Brilliant SYBR Green QPCR Mix (Stratagene, Amsterdam, The Netherlands) in a Mx3000p detection system (Stratagene). Each sample was analyzed in duplicate along with standard and no-template controls. The PCR parameters were 95 ° C for 10 min, 40 cycles at 95 ° C for 15 s, 60 ° C for 1 min and 72 ° C for 30 s. RNA concentrations were assessed by determining cycle threshold (CT) for each sample and subsequently using the 2 – CT method. RNA levels were further corrected with the 18S gene cDNA signal for variations in amounts of input RNA.
Histological Studies. Skin explants were treated by topical application of the ROL product and incubated for 48 h. For Masson trichrome staining, skin explants were fixed in Bouin solution and embedded in paraffin. Ten-micrometer-thick sections were cut using a microtome, and routine Masson trichrome staining was performed. For Ki-67 immunostaining, skin explants were immersed in liquid nitrogen and cut using a Kryostat. Seven-micrometer-thick sections were fixed in acetone, rinsed in PBS and incubated in anti-Ki-67 antibodies (Zymed Laboratories, Clinisciences, Montrouge, France) diluted 1/100 for 2 h. Skin sections were then rinsed and incubated in biotinylated goat anti-mouse immunoglobulins diluted 1:2,500 for 1 h. After incubation in streptavidin-horseradish peroxidase solution for 1 h, staining was revealed using 3,3 -diaminobenzidine. Finally, slides were mounted and coverslipped until cell counting.
Clinical Study
A clinical double-blinded placebo-controlled study was conducted in the period between January and October 2006. The clinical investigation was conducted according to the declaration of Helsinki principles and under the control of a dermatologist who approved the protocol and reviewed the results. The participating volunteers were 48 Caucasian women from the Paris area with ages between 41 and 60 years and skin types I–IV according to Fitzpatrick’s classification. The volunteers were in good health without any skin conditions. Each volunteer signed an informed consent before participating in the study. Before the beginning of the test, they agreed to spend 3 days without any cosmetic products (except cleansers) and 30 days without any antiaging product on the face. They were given a moisturizing product for use during this 30-day washout period.
The volunteers were assigned randomly to each of the two treatment groups (24 volunteers/group), and they were instructed to apply the assigned test product once a day (in the morning) to the whole face under normal conditions of use for the period of the study (36 weeks). Moreover, they were instructed not to apply the test products on the morning of the measurements. The volunteers were allowed to use sunscreen products during the study period to protect their skin from solar UV radiation. No other cosmetic product (except cleansers) was used on the face for the duration of the study.
Skin evaluations were performed at baseline (T0) and at 12 (T1), 18 (T2), 24 (T3) and 36 (T4) weeks of product use. At each visit and before the skin evaluations, the volunteers were acclimated in a temperature- and humidity-controlled room at 20 8 2 ° C and 50 8 5% relative humidity for at least 15 min before each measurement. The skin evaluations included: (a) in vivo assessment of epidermal cell proliferation using fluorescence spectroscopy, (b) clinical assessment by an expert grader, (c) high-resolution digital imaging of the face (front and profile), and (d) three dimensional profilometry of the crow’s feet area.
Fluorescence Measurements. In vivo fluorescence spectroscopy was performed using a SPEX Skin Skan spectrofluorimeter (JY Horiba, Edison, N.J., USA). The excitation source was a xenon arc lamp. A detailed description of the instrumentation is given elsewhere [15] . Measurements were performed by placing the fiberoptic probe in contact with the skin site of interest. Before each set of measurements, the instrument was spectrally calibrated for excitation and emission in the region of 250–650 nm. The chromatic resolution of the spectrofluorimeter was 8 2 nm (provided by the manufacturer). A synchronous scan of excitation and emission was used with 50 nm offset, followed by a synchronous scan with 0 nm offset. The first scan was used to record the skin fluorescence with excitation in the range of 250–500 nm. This scan goes through the major fluorescence maxima naturally occurring in skin with excitations at: (a) 295 nm, attributed to tryptophan moieties and related to epidermal cell proliferation and epidermal thickening [11, 12, 16, 17] ; (b) 335 nm, attributed to pepsin-digestible collagen crosslinks; (c) 370 nm, attributed to collagenase-digestible collagen crosslinks, and (d) 400–420 nm, attributed to elastin crosslinks. For a review of the skin fluorescence maxima see Kollias et al. [18] . The second scan corresponds to acquisition of a diffuse reflectance spectrum and was used to normalize the fluorescence spectrum, in order to account for variations in skin native pigmentation (skin pigmentation attenuates the detected fluorescence signal) [15, 19] . The correction was necessary especially for wavelengths 1 315 nm. The tryptophan fluorescence signal was normalized to the 390 nm excitation band following the recommendation given in Stamatas et al. [20] . Apart from taking into account artifacts due to native pigmentation, normalization of the signals also minimizes the effect of instrumental parameters on the measurements.
At all time points skin fluorescence measurements were performed on the central cheek area, 2 cm below the cheekbone. To avoid left-right side bias, the volunteers were randomized for the side of measurement. The same side was used in a consistent way for each volunteer for all time points of measurements. The fluorescence of 0.1% ROL at 295 nm excitation was examined and found not to interfere with the natural skin fluorescence at this excitation wavelength (data not shown).
Clinical Assessment. Clinical assessment was performed for wrinkles (crow’s feet area, area below the eyes), fine lines (crow’s feet area), brown spots and skin tone evenness using a 12-cm visual analog scale. The results of the clinical assessment are given as percent improvement from baseline.
Digital Imaging. Pictures were acquired using a standardized setup that has been developed by Johnson & Johnson (United States Patent 6907193). The system allows taking high-resolution facial pictures in a controlled lighting and positioning environment in a standardized way. In brief, high-resolution digital imaging was performed in a dark room using a Nikon D100 camera (Nikon Inc., Melville, N.Y., USA) with a 60-mm objective. The camera was mounted on a custom-made setup with a chin rest for positioning of the volunteers. The geometry of the setup was designed to optimize the coverage of the acquired image by the face of the person being imaged. A color standard was mounted in front of the chin rest and was included in the field of view so that color reproducibility could be verified. Illumination was provided by an external flat light-emitting diode flash unit synchronized with the camera shutter and fully calibrated so that the control of the light source allows having comparable colors across time points. A front face image was first acquired followed by a profile image with the chin rest rotated by 60° with respect to the camera. The acquisition software allowed for viewing of the baseline image for optimal repositioning of the subject.
Profilometry. Three-dimensional profilometry was performed using an Optotop Sensor (Eotech, Marcoussis, France) mounted on a Visioface bench (Eotech). The setup was optimized for acquiring surface profiles of the crow’s feet area. The acquisition software allowed for comparison of the current with a previous profile for optimal repositioning of the subject.
Table 1. ROL improves under-eye wrinkles, fine crow’s feet lines and skin tone evenness
Parameters | Time, weeks | Placebo | Active agent |
|---|---|---|---|
Wrinkles under the eyes | baseline | 4.482.3 | 4.981.8 |
Fine lines | baseline | 4.981.9 | 4.982.3 |
Skin tone evenness | baseline | 4.981.7 | 4.981.5 |
Clinical assessment was performed on a 12-cm linear analog scale by an expert grader. NS = No significant difference from baseline; a p < 0.05, b p < 0.01, c p < 0.001 compared to baseline; d p < 0.05, e p < 0.01, compared to placebo.
Statistics
For the in vitro study, results are expressed as means 8 1 standard error of the mean (SEM). For group comparisons, Student’s t test followed by the Mann-Whitney test was performed using Graph Pad software. Significance was considered for p ! 0.05. For the clinical study, mean values and standard deviations were calculated for all the subjects of each treated group and at each time point. Group comparisons were assessed using Student’s t test as all data are normally distributed (normality was tested using the Anderson-Darling method). Comparisons were made: (a) between each time point and baseline for each group and (b) between treatments (active vs. placebo) for each time point. Significance was considered for p ! 0.05.
Results
Clinical Results: 0.1% ROL Is Improving Fine Line Appearance and Skin Tone Evenness The long-term application of the product containing 0.1% ROL did not trigger any significant irritation. Clinical assessment of wrinkles under the eyes, fine lines in the crow’s feet area and skin tone evenness showed that all of these parameters improved for the active-agent treated group by the first measurement (12 weeks of use; table 1 ). For the fine lines and skin tone evenness there was significant improvement compared to baseline for the placebo-treated group, which can possibly be attributed to a moisturizing effect of the applied product. However, at most time points there was significant improvement of the assessed parameters in the actively treated group compared to the placebo treatment. The improvement of the fine lines in the crow’s feet area was also recorded with digital imaging and with surface profilometry. A representative image of the observed visible improvements in the appearance of fine lines during the ROL treatment is shown in figure 1 . A progressive improvement can be seen between baseline and 24 and 36 weeks of treatment. The improvement in fine lines was also documented by surface profilometry. A representative image is shown in figure 2 . The progressive disappearance of the fine lines in the crow’s feet area can be observed. Ex vivo Results: 0.1% ROL Increases the Expression of Epidermal Genes CRABP2 and HBEGF The ex vivo activity of ROL on skin explants was evaluated by measuring the effect of ROL on gene expression by means of real-time QPCR. Two key genes, relevant for retinoid-like activity, were chosen, namely those of cellular retinoic-acid-binding protein II (CRABP2) and heparin-binding epidermal growth factor (HBEGF). Application of 0.1% ROL onto skin explants for 24 and 48 h produced a significant increase in CRABP2 mRNA expression. This increase reached +388.8% after 24 h and +932% after 48 h of incubation ( fig. 3 ). Formulated ROL also caused a significant increase in HBEGF gene expression. Indeed, as shown in figure 4 , ROL significantly stimulated the expression of HBEGF transcripts after 24 h of application (+184.3%) and after 48 h of application (+318.3%; fig. 4 ).
Ex vivo Results: 0.1% ROL Increases the Proliferation of Epidermal Cells and Epidermal Thickness By performing Masson trichrome staining, we have shown that application of 0.1% ROL for 2 days visibly increased epidermal thickness when compared to untreated control ( fig. 5 ). In addition, immunohistochemical staining specific for Ki-67, a cell cycle protein, revealed that ROL application actually increased the number of proliferating cells in skin explants (+119.3%; fig. 6 ).
Clinical Results: 0.1% ROL Increases Epidermal Cell Proliferation in vivo The epidermal cell proliferation rate (turnover rate) was evaluated in the 36-week clinical study using in vivo
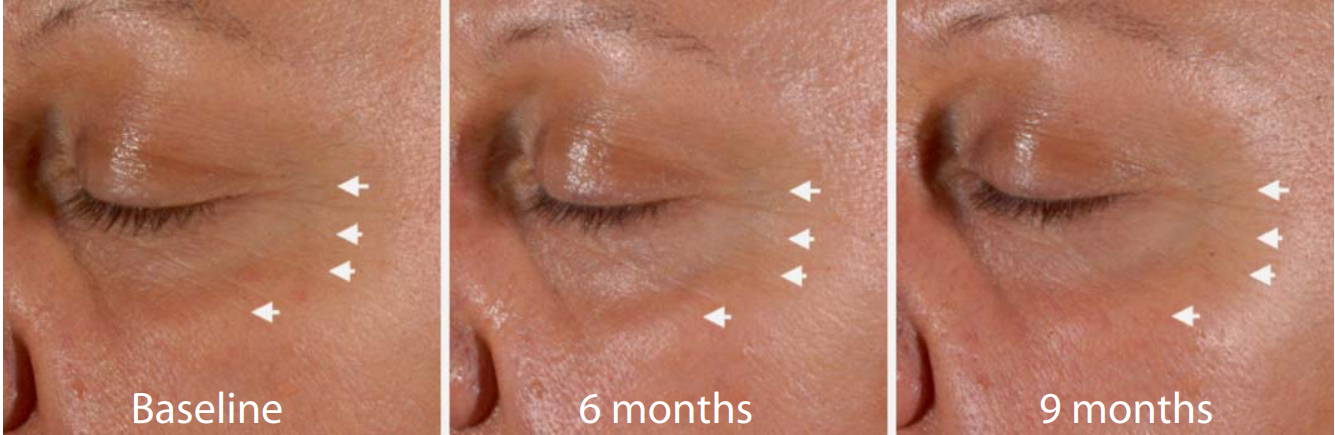
Fig. 1. ROL improves the visible appearance of fine lines. Representative images of a volunteer treated with 0.1% ROL at baseline, 24 and 36 weeks. Arrows point to fine lines that improve their appearance in the course of the study.
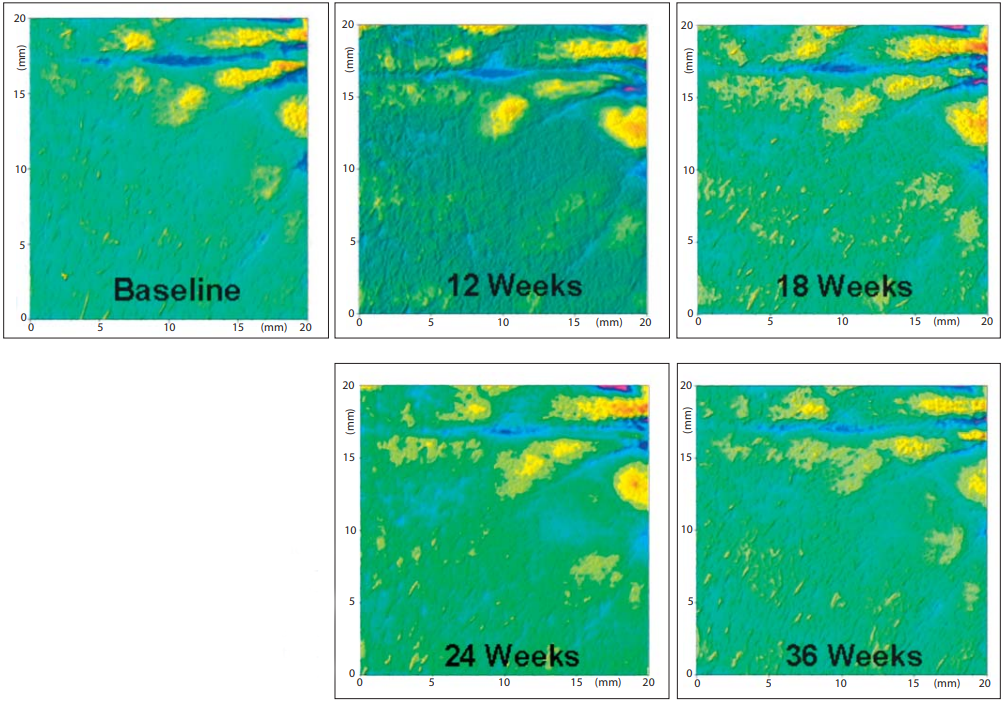
Fig. 2. ROL improves the three-dimensional appearance of fine lines. Representative surface profilometry images demonstrating the three-dimensional appearance of fine lines of a volunteer treated with 0.1% ROL in the course of the study. The imaged area corresponds to the crow’s feet region on the side of the eyes.
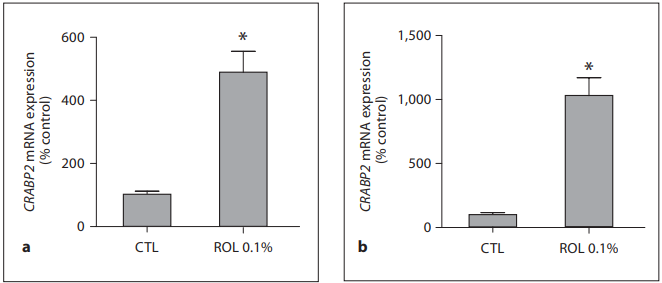
Fig. 3. 0.1% ROL upregulates CRABP2 gene expression. ROL at a concentration of 0.1% was applied onto skin explants for 24 h ( a ) and 48 h ( b ). Epidermis samples were collected, and RNAs were extracted and reverse transcribed. Real-time QPCR was performed using a primer pair specific for CRABP2 gene, and the amount of target transcripts was normalized using an 18S RNA normalization gene. Data are expressed as percentage of control (CTL, untreated control = 100%) and are presented as means 8 SEM (n = 4). * p ! 0.05 versus untreated control.
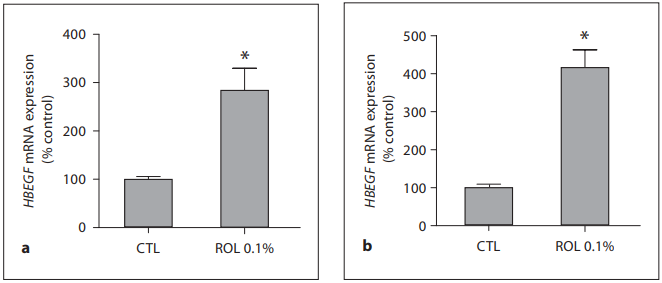
Fig. 4. 0.1% ROL upregulates HBEGF gene expression. ROL at a concentration of 0.1% was applied onto skin explants for 24 h ( a ) and 48 h ( b ). Epidermis samples were collected, and RNAs were extracted and reverse transcribed. Real-time QPCR was performed using a primer pair specific for HBEGF gene, and the amount of target transcripts was normalized using an 18S RNA normalization gene. Data are expressed as percentage of control (CTL, untreated control = 100%) and are presented as means 8 SEM (n = 4). * p ! 0.05 versus untreated control.
fluorescence spectroscopy by measuring the fluorescence maximum attributed to tryptophan moieties. After normalization for variances in native pigmentation, the placebo-treated group did not show any significant change from baseline for any time point ( fig. 7 ). In contrast, the intensity of tryptophan fluorescence showed an increase for the actively treated group. The increase in fluorescence in the actively treated group is evident from the first time point (12 weeks vs. baseline) and then it appears to reach a plateau. Most importantly, the change in the fluorescence intensity from baseline was significantly higher for the active-agent-treated group than for the placebo-treated group at the 36-week time point.
Discussion
Many studies have established that ATRA shows clinical effectiveness in treating skin photoaging. It has also been reported that ROL, a less irritating retinoid, at doses of 0.4%, can reduce chronological aging signs. However, it is now well documented that ROL formulated at high doses in a product can trigger irritation responses in
the skin. In the present study, we have investigated the effect of topical applications of ROL at a low and tolerable dose (0.1%) ex vivo on skin explants and in vivo on volunteers. Because clinical effects of ROL on photoexposed skin sites are poorly described, we focused our clinical study on face skin. 0.1% ROL Increases HBEGF and CRABP2 Expression and Stimulates Keratinocyte Proliferation in vitro Previous studies have shown that CRABP2 is induced by ATRA in full-thickness skin [21] , in cultured fibroblasts [22] and in the epidermis [8] . In fact, CRABP2 has been suggested to be a key marker of retinoid-like activity in the skin [21] . Our results show that topical application of a formulation containing 0.1% ROL was able to increase CRABP2 expression in the epidermis. These results indicate that even at low concentrations, ROL could exert an efficacious and quantifiable retinoid-like activity. Our data also demonstrate that 0.1% ROL drastically stimulates HBEGF gene expression in explant epidermis. HBEGF is an autocrine growth factor that is involved in the effects of retinoids on epidermal hyperplasia. In par
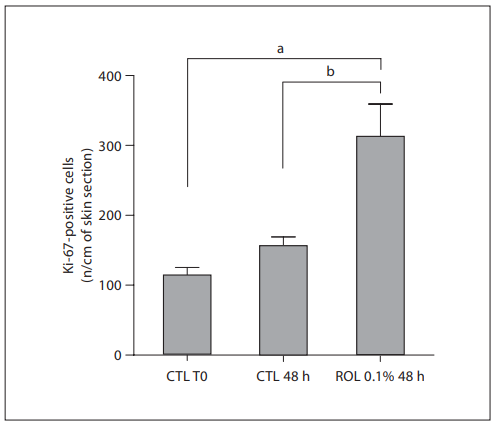
Fig. 5. ROL-induced increase in the number of epidermal proliferating cells in human skin explants. ROL at a concentration of 0.1% was applied onto skin explants for 48 h. Epidermal proliferating cells were visualized by immunohistochemical staining of Ki-67 protein, after 48 h of application. Positive cells were counted (3 counts/explant). Data are expressed as the mean number 8 SEM (n = 3 counts) of positive cells per centimeter of skin section. a p ! 0.01 versus untreated control at T0; b p ! 0.05 versus untreated control at 48 h. CTL = Control.
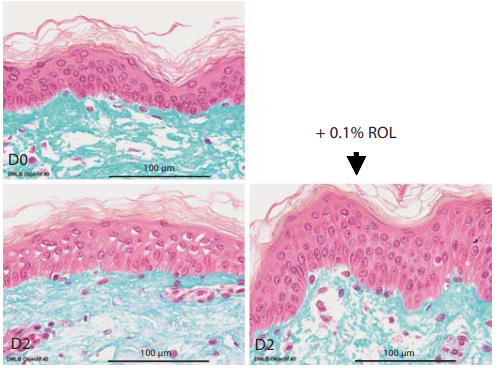
Fig. 6. ROL-induced increase in epidermal thickness ex vivo in human skin explants. ROL at a concentration of 0.1% was applied onto skin explants for 48 h. Skin histology was visualized on day 0 (D0) and day 2 (48 h of incubation, D2) after Masson trichrome staining of the explants.
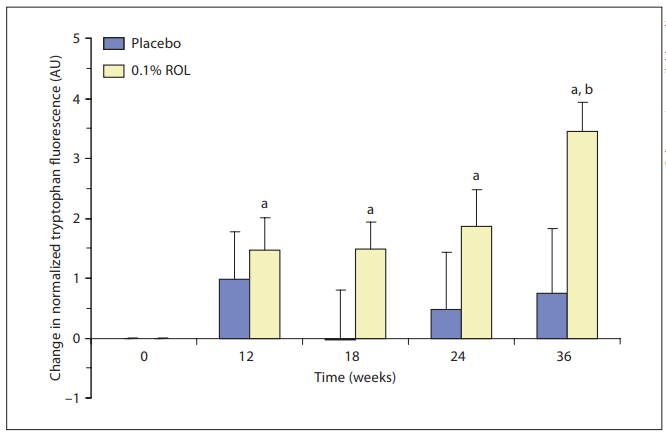
Fig. 7. ROL-induced increase in epidermal cell proliferation measured in vivo. This chart shows the changes from baseline of the normalized tryptophan fluorescence for each treated group and for each time point. A positive change reflects an increase in epidermal cell proliferation. a p ! 0.05: significant difference from baseline values; b p ! 0.05: significant difference between an active-agent-treated group and the placebo-treated group.
ticular, it has been shown that HBEGF expression is induced by retinoids in mouse skin [23] , and the effects of retinoids on keratinocyte proliferation are inhibited by antibodies directed against HBEGF as well as by antagonists of c-ErbB receptor which is specific for HBEGF [24] . In the present study, we demonstrate that 0.1% ROL can increase HBEGF gene expression. This effect was accompanied by an increase in the number of Ki-67-positive cells together with a thickening of the epidermis as revealed in our histological study. Therefore, it is conceivable that our cosmetic formulation containing low amounts of ROL could induce cell proliferation and hence epidermal hyperplasia by induction of HBEGF.
0.1% ROL Increases Cell Proliferation in vivo It has been demonstrated that fluorescence spectroscopy can be used as an objective quantitative method for studying skin aging and photoaging [11–14, 20, 25, 26] . The fluorescence due to tryptophan moieties decreases with age, implying an age-related reduction of the epidermal cell turnover rate, both in SKH mice [11] and in human skin [20] . Tryptophan fluorescence is an ideal marker for noninvasive monitoring of the epidermal cell proliferation rate [17] . Increases in this fluorescence signal have been measured following interventions that are known to augment epidermal proliferation [11, 12, 16] . Our results demonstrate that 0.1% ROL significantly increases cell proliferation in vivo ( when compared to baseline) as early as 12 weeks following the beginning of the clinical trial. However, when compared to placebo, 0.1% ROL induces a significant stimulatory effect only after 36 weeks of product usage. It should be noted that fluorescence data for the placebo-treated group were remarkably variable, as shown by error bars, while fluorescence data collected in the actively treated group were more evenly distributed, suggesting that ROL may standardize cell proliferation among volunteers. Therefore, although the fluorescence signal tends to be visibly higher in the actively treated group, significance could be reached only at the last time point investigated in this study, when the effect of ROL is maximal. On the other hand, we cannot rule out the possibility that the fluorescence signal needs to accumulate over time before the stimulatory effect of 0.1% ROL reaches significance in vivo. Ex vivo, we demonstrated that 0.1% ROL induced a proliferation of epidermal cells as early as 48 h after topical application while in vivo we observed the first signs of improvement after 12 weeks of application. This may reflect the sensitivity of the explant model to measure biological activity of topical products. Indeed, since
skin biopsies are devoid of blood and fluid circulation, ROL may thus accumulate within the epidermis, leading to an enhanced stimulatory activity of the applied product. We also observed that the tryptophan fluorescence signal markedly increases at the last time point (36 weeks). This observation may be explained by taking into account that this measurement was performed in October, following a season when the skin has been repeatedly challenged by solar UV rays. The skin responds to such environmental insults by an innate repair mechanism that involves induction of keratinocyte hyperproliferation [27] . It has been demonstrated that these innate repair mechanisms of facial skin, i.e. its ability to maintain high proliferation rates following external aggressions, are gradually compromised with age [20] . It is conceivable that topical treatment with ROL is actually restoring the ability of the skin to respond to environmental insults contrary to the placebo-treated group for which the signal remained unchanged.
0.1% ROL Decreases Signs of Aging We have observed that 3 different signs of aging have been improved with treatment of 0.1% ROL. The 3 examples of aging signs that improved show the action of ROL at 3 different levels. Epidermal thinning, with retraction of rete ridges, is a hallmark of aging skin [1, 28] , and the epidermis is particularly thin at the bottom of the wrinkles and fine lines [29] . The observed ROL-induced improvement of the appearance of fine lines is expected to be due to an increase in epidermal thickening (demonstrated by the results of the ex vivo study), which in turn is the result of ROL stimulation of epidermal cell proliferation (demonstrated both by the ex vivo and the in vivo results). This is supported by the finding that there is a significant correlation between the increase from baseline in the normalized tryptophan fluorescence signal and the clinical improvement of fine lines (R = 0.59, p ! 0.01). In other words, subjects with higher responses to ROL-induced increase in proliferation were also the people with higher improvement in fine lines. The second clinical parameter that ameliorated with ROL treatment, wrinkles under the eyes, is expected to be related to secondary effects of ROL on the dermal layer. It has been hypothesized that retinoids could increase collagen synthesis either by restoring procollagen expression by inhibition of c-Jun signaling which is triggered by UV irradiation [30] or by activation of TGF- -mediated signal transduction [31] .
We demonstrate that low doses of ROL could alleviate wrinkles under the eyes, which is typically related to a restoration of extracellular matrix. Because the amount of ROL applied to the skin was very low in the present study, it seems unlikely that ROL treatment would induce direct effects in the dermis. Actually, it is more conceivable that a ROL formulation would exert primary effects in the epidermis and induce the release of TGF- by keratinocytes, which may in turn induce collagen synthesis in dermal fibroblasts by a paracrine action. In addition, the antiaging action of ROL at low concentrations may be consecutive to its effect on the oxidative status of the skin. In support of this hypothesis, a number of reports have shown that low concentrations of antioxidant substances such as carotenoids and vitamins are able to decrease skin damage induced by UV, thereby limiting the deleterious effects of oxidative stress on skin aging [32] . Finally, we observed that ROL treatment improved the appearance of skin tone uniformity. Photoaging is most often associated with pigmentation disorders such as mottled hyperpigmentation and solar lentigines [33] that are consecutive to a UV-induced dysregulation of melanocytic function and melanin transfer to keratinocytes [34] . Retinoids have been shown to reduce hyperpigmentation and normalize pigmentation levels [35] , presumably by inhibiting melanosome transfer and decreasing the melanin content by the enhancement of epidermal turnover [36] . Therefore, it seems likely that 0.1% ROL may normalize the pigmentation level and decrease the occurrence of hyperpigmented skin lesions, thereby improving the appearance of skin tone uniformity.
ROL Concentration A previous study has shown that 0.2% ROL activates specific proinflammatory cytokines [9] . Therefore, we preferred to test ROL at a concentration of 0.1% in order to determine whether a lower concentration of ROL could still induce a beneficial effect on skin aging signs without inducing any adverse reaction. To our knowledge this is the first study to demonstrate ROL-induced antiaging effects at 0.1%. Moreover, in our 36-week study there were no adverse reactions in the ROL panel, confirming the absence of unwanted side effects of ROL at 0.1%. In conclusion, we have demonstrated that ROL at a concentration of 0.1% ex vivo could induce CRABP2 gene expression, thereby showing a retinoid-like activity. We also demonstrated both ex vivo, by assessing HBEGF gene expression, and in vivo, as revealed by tryptophan fluorescence measurement, that the ROL product promoted epidermal cell proliferation. These effects were accompanied by a significant improvement of wrinkles under the eyes, fine lines and uneven tone as shown by our clinical investigation. In turn, these results strongly suggest that the beneficial effect of ROL at a low concentration may be consecutive, at least in part, to its effect in the epidermis. Altogether, these data show that a topical product containing 0.1% ROL could successfully alleviate major aging signs without any significant adverse skin reaction. Acknowledgements We gratefully thank Anne Sophie Brillouet for product formulation and Stan Shapiro for expert review of the paper.
References
1 Baumann L: Skin ageing and its treatment. J Pathol 2007;211:241–251.
2 Griffiths CE, Russman AN, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ: Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid). N Engl J Med 1993;329:530–535.
3 Griffiths CE, Kang S, Ellis CN, Kim KJ, Finkel LJ, Ortiz-Ferrer LC, White GM, Hamilton TA, Voorhees JJ: Two concentrations of topical tretinoin (retinoic acid) cause similar improvement of photoaging but different degrees of irritation: a double-blind, vehiclecontrolled comparison of 0.1% and 0.025% tretinoin creams. Arch Dermatol 1995;131: 1037–1044.
4 Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ: Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996;379: 335–339.
5 Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ: Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med 1997;337:1419–1428.
6 Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher GJ, Voorhees JJ: Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin
7. Kafi R, Kwak HS, Schumacher WE, Cho S, Hanft VN, Hamilton TA, King AL, Neal JD, Varani J, Fisher GJ, Voorhees JJ, Kang S: Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol 2007;143: 606–612.
8 Kang S, Duell EA, Fisher GJ, Datta SC, Wang ZQ, Reddy AP, Tavakkol A, Yi JY, Griffiths CE, Elder JT, et al: Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J Invest Dermatol 1995;105:549– 556.
9 Kim BH, Lee YS, Kang KS: The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol Lett 2003;146:65–73.
10 Kollias N, Stamatas GN: Optical non-invasive approaches to diagnosis of skin diseases. J Invest Dermatol Symp Proc 2002;7:64–75.
11 Kollias N, Gillies R, Moran M, Kochevar IE, Anderson RR: Endogenous skin fluorescence includes bands that may serve as quantitative markers of aging and photoaging. J Invest Dermatol 1998;111:776–780.
12 Brancaleon L, Lin G, Kollias N: The in vivo fluorescence of tryptophan moieties in human skin increases with UV exposure and is a marker for epidermal proliferation. J Invest Dermatol 1999;113:977–982.
13 Leffell DJ, Stetz ML, Milstone LM, Deckelbaum LI: In vivo fluorescence of human skin: a potential marker of photoaging. Arch Dermatol 1988;124:1514–1518.
14 Na R, Stender IM, Henriksen M, Wulf HC: Autofluorescence of human skin is age-related after correction for skin pigmentation and redness. J Invest Dermatol 2001;116: 536–540.
15 Stamatas GN, Wu J, Kollias N: Non-invasive method for quantitative evaluation of exogenous compound deposition on skin. J Invest Dermatol 2002;118:295–302.
16 Doukas AG, Soukos NS, Babusis S, Appa Y, Kollias N: Fluorescence excitation spectroscopy for the measurement of epidermal proliferation. Photochem Photobiol 2001;74:96– 102.
17 Zhang JC, Savage HE, Sacks PG, Delohery T, Alfano RR, Katz A, Schantz SP: Innate cellular fluorescence reflects alterations in cellular proliferation. Lasers Surg Med 1997;20: 319–331.
18 Kollias N, Zonios G, Stamatas GN: Fluorescence spectroscopy of skin. Vib Spectrosc 2002;28:17–23.
19 Wu J, Feld MS, Rava RP: Analytical model for extracting intrinsic fluorescence in turbid media. Appl Opt 1993;32:3585–3595.
20 Stamatas GN, Estanislao RB, Suero M, Rivera ZS, Li J, Khaiat A, Kollias N: Facial skin fluorescence as a marker of the skin’s response to chronic environmental insults and its dependence on age. Br J Dermatol 2006; 154:125–132.
21 Elder JT, Cromie MA, Griffiths CE, Chambon P, Voorhees JJ: Stimulus-selective induction of CRABP-II mRNA: a marker for retinoic acid action in human skin. J Invest Dermatol 1993;100:356–359.
22 Elder JT, Kaplan A, Cromie MA, Kang S, Voorhees JJ: Retinoid induction of CRABP II mRNA in human dermal fibroblasts: use as a retinoid bioassay. J Invest Dermatol 1996; 106:517–521.
23 Chapellier B, Mark M, Messaddeq N, Calleja C, Warot X, Brocard J, Gerard C, Li M, Metzger D, Ghyselinck NB, Chambon P: Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. Embo J 2002;21:3402– 3413.
24 Varani J, Zeigler M, Dame MK, Kang S, Fisher GJ, Voorhees JJ, Stoll SW, Elder JT: Heparin-binding epidermal-growth-factor-like growth factor activation of keratinocyte ErbB receptors mediates epidermal hyperplasia, a prominent side-effect of retinoid therapy. J Invest Dermatol 2001;117:1335– 1341.
25 Sandby-Moller J, Thieden E, Philipsen P, Heydenreich J, Wulf H: Skin autofluorescence as a biological UVR dosimeter. Photodermatol Photoimmunol Photomed 2004; 20:33–40.
26 Tian WD, Gillies R, Brancaleon L, Kollias N: Aging and effects of ultraviolet A exposure may be quantified by fluorescence excitation spectroscopy in vivo. J Invest Dermatol 2001; 116:840–845.
27 Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ: Mechanisms of photoaging and chronological skin aging. Arch Dermatol 2002;138:1462–1470.
28 Smith L: Histopathologic characteristics and ultrastructure of aging skin. Cutis 1989;43: 414–424.
29 Contet-Audonneau JL, Jeanmaire C, Pauly G: A histological study of human wrinkle structures: comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br J Dermatol 1999;140:1038–1047.
30 Fisher GJ, Datta S, Wang Z, Li XY, Quan T, Chung JH, Kang S, Voorhees JJ: c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all- trans retinoic acid. J Clin Invest 2000;106:663–670.
31 Kim HJ, Bogdan NJ, D’Agostaro LJ, Gold LI, Bryce GF: Effect of topical retinoic acids on the levels of collagen mRNA during the repair of UVB-induced dermal damage in the hairless mouse and the possible role of TGFbeta as a mediator. J Invest Dermatol 1992; 98:359–363.
32 Darvin M, Zastrow L, Sterry W, Lademann J: Effect of supplemented and topically applied antioxidant substances on human tissue. Skin Pharmacol Physiol 2006;19:238– 247.
33 Stratigos AJ, Katsambas AD: The role of topical retinoids in the treatment of photoaging. Drugs 2005;65:1061–1072.
34 Costin GE, Hearing VJ: Human skin pigmentation: melanocytes modulate skin color in response to stress. Faseb J 2007;21:976– 994.
35 Griffiths CE, Goldfarb MT, Finkel LJ, Roulia V, Bonawitz M, Hamilton TA, Ellis CN, Voorhees JJ: Topical tretinoin (retinoic acid) treatment of hyperpigmented lesions associated with photoaging in Chinese and Japanese patients: a vehicle-controlled trial. J Am Acad Dermatol 1994;30:76–84. 36 Ortonne JP: Retinoid therapy of pigmentary disorders. Dermatol Ther 2006;19:280–288.