NicoDerm® Patch
Continuous control over 24 hours1
Discover how to use NicoDerm® as part of a proven quit strategy such as: REDUCE TO QUIT®, pre-cessation or combination therapy.
Can be used as part of the REDUCE TO QUIT® program
Can be used to quit immediately or for pre-cessation treatment
Provides a 3-step program to reduce nicotine cravings1
Discreet and transparent
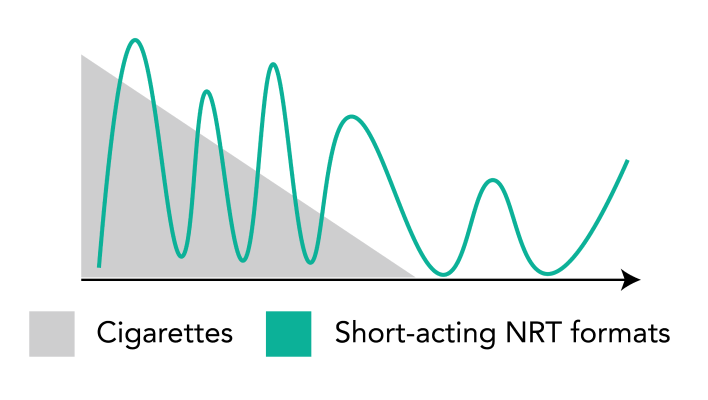
Reduces withdrawal symptoms and cravings — night and day (including early morning cravings)
Provides patients with a steady, measured flow of nicotine over 24 hours
Water won’t harm the NicoDerm® Patch while being worn3
* In a randomized, double-blind, placebo-controlled clinical trial, 513 smokers with a smoking history of at least 1 pack a day were randomized to receive NICODERM® 21 mg/day, 14 mg/day, 7 mg/day or placebo. Patients received smoking cessation counselling during the 6-week course of the trial.↩

For complete directions for use and warnings, always refer to the product insert/booklet provided with the product.
Step 1 | Weeks 1–6 | 21 mg | One Patch daily |
Step 2 | Weeks 7–8 | 14 mg | One Patch daily |
Step 3 | Weeks 9–10 | 7 mg | One Patch daily |
For patients who smoke <10 cigarette/day, weigh less than 45 kg (100 lbs) or have cardiovascular disease:
Start with Step 2 (14 mg) one Patch/day for 6 weeks
Then, use Step 3 (7 mg) one Patch/day for 2 to 4 weeks then stop
Reassess your patient if they need to use the NicoDerm® Patch for longer than 6 months to keep from smoking.
It is important to complete treatment. If your patient has a cigarette while trying to quit smoking, instruct them to keep using the NicoDerm® Patch and keep trying to quit.
If the NicoDerm® Patch does not help your patient stop smoking, reassess them and explore about using more than one type of nicotine replacement therapy at the same time.
Cut package along dotted line, remove the Patch and remove liner from patch
On a dry hairless area above the waist or on the upper outer area of the arm, back or shoulders, press Patch down firmly for 10 seconds (wash hands without soap)
After 24 hours, replace the Patch with a new one. If sleep is disrupted, it can be removed before bedtime.
To ensure safe disposal, used patches should be discarded by folding them with the sticky sides together to minimize risk to children and pets.
Keep out of reach of children.
DO NOT USE IF YOUR PATIENT IS/HAS: under 18 years of age; an occasional or non-smoker; pregnant or nursing; generalized skin disorders (e.g., eczema, rash; allergic to nicotine or any of the non-medicinal ingredients).
USE WITH CAUTION IF YOUR PATIENT: has/had heart disease, a heart attack, heart pain, irregular heartbeat or a stroke or have ever had high blood pressure, blood circulation problems or treatment for circulation disorders of the brain, asthma, chronic lung problems, thyroid problems, stomach problems, ulcers or inflammation of the esophagus, kidney or liver disease, history of epilepsy or seizures, diabetes requiring insulin or are taking any prescription medications.
Important
Do not use more than one Patch at a time, as this may cause nicotine overdose.
Remove the NicoDerm® Patch 2 hours before engaging in prolonged, strenuous exercise, as this may increase nicotine absorption through the skin and cause symptoms of nicotine overdose.
For product support, call 1-866-311-5869.
Medicinal Ingredients:
Each NicoDerm® Step 1 (21 mg/day) Patch contains 114 mg nicotine. Each Patch releases a controlled 1.5 mg/hr over 24 hours with systemic delivery of 21 mg/day.
Each NicoDerm® Step 2 (14 mg/day) Patch contains 78 mg nicotine. Each Patch releases a controlled 1.0 mg/hr over 24 hours with systemic delivery of 14 mg/day.
Each NicoDerm® Step 3 (7 mg/day) Patch contains 36 mg nicotine. Each Patch releases a controlled 0.5 mg/hr over 24 hours with systemic delivery of 7 mg/day.
Non-Medicinal Ingredients: Ethylene-vinyl acetate copolymer, polyisobutylene and high-density polyethylene between clear polyester backings.
Did you know? Combination NRT therapy is 30% more effective vs NRT monotherapy in helping patients to quit successfully.1 Increase your patient’s chance of success with combination NRT therapy.
Step-by-step: how to use NicoDerm® Patch
Smoking cessation strategies
Discover how to use the NicoDerm® Patch as part of a proven quit strategy such as REDUCE TO QUIT®, pre-cessation or combination therapy.
Related products
References
1. NICODERM® Product License. February 16, 2016.
2. Transdermal Nicotine Study Group. Transdermal Nicotine for Smoking Cessation – Six-Month Results From Two Multicenter Controlled Clinical Trials. JAMA, December 11, 1991 – Vol 266, No. 22. Available at: http://jama.jamanetwork.com/article.aspx?articleid=393805.
3. NICODERM® Product Insert