What is nicotine replacement therapy (NRT)?
Nicotine replacement therapy (NRT) works by providing a lower amount of nicotine to help relieve cravings and tobacco withdrawal symptoms.
NRT delivers nicotine differently than cigarettes
NRT stabilizes nicotine levels in the blood3
To help reduce withdrawal from faster-acting nicotine delivery mechanisms such as, cigarettes.

NRT has a slower nicotine delivery mechanism3
This can be less reinforcing when compared with the explosively rapid delivery of nicotine from cigarettes, which can deliver to the pulmonary system in 10-20 seconds.
Effectiveness and safety of NRT for achieving long-term smoking cessation vs placebo, 6 months or longer follow up1
Type of NRT | RR | 95% CI | I2 | N of studies | N of participants |
|---|---|---|---|---|---|
Gum | 1.49 | 1.40 to 1.60 | 40% | 56* | 10,596 / 11,985 |
Patch | 1.64 | 1.53 to 1.75 | 24% | 51 | 13,773 / 11,981 |
Inhalator | 1.90 | 1.36 to 2.67 | 0% | 4 | 490 / 486 |
Intranasal spray | 2.02 | 1.49 to 2.73 | 0% | 4 | 448 / 439 |
Tablets/lozenges | 1.52 | 1.32 to 1.74 | 71% | 8* | 2326 / 2113 |
Oral spray | 2.48 | 1.24 to 4.94 | N/A | 1 | 318 / 161 |
Choice of product | 1.37 | 1.25 to 1.52 | 42% | 7 | 4179 / 4109 |
Patch and inhalator | 1.07 | 0.57 to 1.99 | NA | 1 | 136 / 109 |
Patch and lozenge | 1.83 | 1.01 to 3.31 | N/A | 1 | 267 / 41 |
Patch and gum | 1.15 | 0.64 to 2.06 | 50% | 2 | 173 / 86 |
Patch, gum and lozenge | 15.00 | 2.00 to 112.54 | N/A | 1 | 212 / 212 |
*includes 1 study treated as 2 for analysis; N/A: not applicable↩
Adapted from Hartmann-Boyce, J, et al.1
Smokers are almost 2x more likely to quit smoking after using a patch vs. placebo.1
Smokers are 3.6x more likely to quit smoking after using a patch + gum or spray vs. placebo.6
Adhering to NRT use can improve the success rate for quitting7
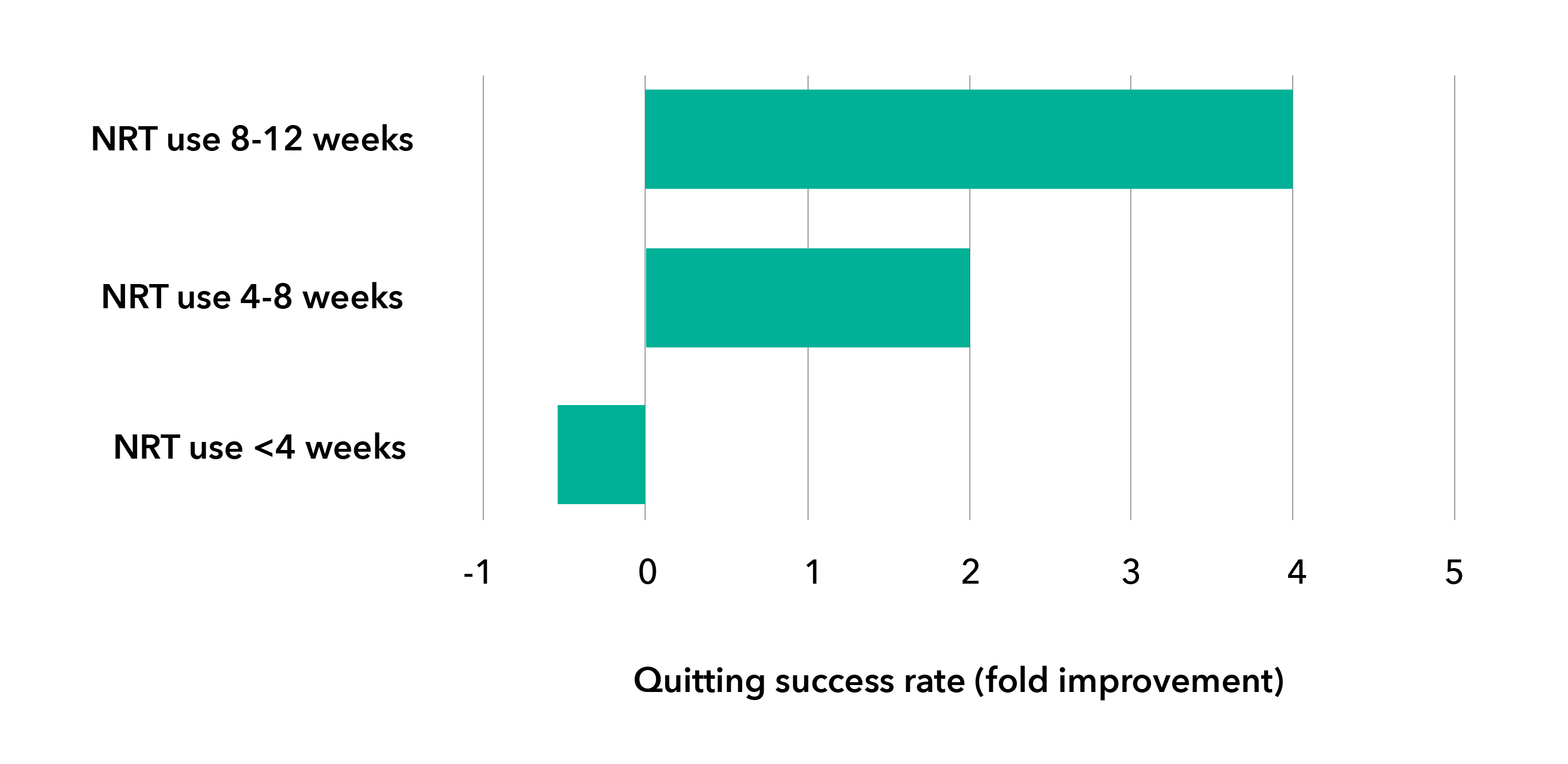
Only 50% or less8 of NRT users adhere to their recommended length of treatment.
Smokers should be encouraged to continue and use appropriate quantities of NRT over a sufficient duration of time (8-12 weeks, or longer duration, if needed, based on professional opinion).7,9
The long-term use of NRT is proven to be safe when used as directed
NRT has been used by millions of patients in over 25 years of clinical use worldwide.10
Safety of the nicotine patch was demonstrated in a randomized, 52-week study11:
Trial Purpose
A randomized trial (N=525) designed to examine the effects of nicotine patch therapy at 8, 24 and 52 weeks on smoking cessation rates.
Interventions
Nicotine patch therapy provided as standard (8 weeks), extended (24 weeks) or long-term treatment (52 weeks). All participants received 12 behavioural counselling sessions.
Main outcomes
The findings support the safety of extended use of nicotine patch treatment:
Extended treatment arm had higher adherence to the nicotine patch vs the standard and maintenance treatment arms (mean, [SD]: 4.7 [2.4] vs 4.61 [2.0] and 3.94 [2.5] patches/week, respectively; F2,522 = 6.03, p = 0.003)
Efficacy was demonstrated in the extended and maintained use of the nicotine patch for 24 weeks vs standard treatment with:
Significantly greater abstinence rates (odds ratio [OR], 1.70 [95%CI, 1.03-2.81]; p = 0.04)
Longer duration of abstinence until relapse (β = 21.30 [95%CI, 10.30-32.25]; P < 0.001)
Fewer cigarettes per day if not abstinent (mean [SD], 5.8 [5.3] vs 6.4 [5.1] cigarettes per day; β = 0.43 [95%CI, 0.06-0.82]; P = 0.02),
More abstinent days (mean [SD], 80.5 [38.1] vs 68.2 [43.7] days; OR, 1.55 [95% CI, 1.06-2.26]; P = 0.02)
Safety of nicotine polacrilex gum was demonstrated in a randomized, 60-week study12:
Trial Purpose
A randomized trial (N=5887) designed to assess cardiovascular conditions and other side effects associated with the use of nicotine polacrilex (NP), 2 mg.
Interventions
Smoking cessation program, including nicotine polacrilex.
Main outcomes
The findings support the safe use of nicotine polacrilex gum as part of a smoking cessation program:
The rates of hospitalization for cardiovascular conditions and cardiovascular deaths during the 5 years of the study were not related to use of NP, to dose of NP, or to concomitant use of NP and cigarettes
About 25% of NP users reported at least one side effect, but most were very minor and transient
Side effects associated with discontinuance of NP in ≥5% or more of users included headache, indigestion, mouth irritation, mouth ulcers, and nausea
There was no evidence that concomitant use of NP and cigarettes was associated with elevated rates of reported side effects
A case with e-cigarettes: Does it help smokers quit?
As e-cigarettes have evolved, their nicotine delivery has improved. This could mean that their addiction potential has increased13
It is not yet clear how addictive e-cigarettes are, or could be, relative to tobacco cigarettes13
The proposed Concentration of Nicotine in Vaping Products Regulations establishes a maximum nicotine concentration of 20 mg/mL for vaping products in Canada to reduce their appeal to youth14
Potential adverse health effects include: throat irritation, increased airway resistance, chronic bronchitis, and transient damage to cardiovascular tissue15,16
The long-term impact of nicotine from e-cigarettes on lung tissue is not yet known and may be different from its impact systemically.
References
1. Hartmann-Boyce, J., Chepkin, S. C., Ye, W., Bullen, C., & Lancaster, T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database of Systematic Reviews. 2018.
2. Sutherland G. Smoking: Can We Really Make a Difference? Heart 2003;89:ii25-7.
3. Flowers L. Nicotine Replacement Therapy. Am J Psychiatry https://psychiatryonline.org/doi/full/10.1176/appi.ajp-rj.2016.110602
4. Instructions for Use, Package Insert, Nicorette®Oral Spray, Data on File, Kenvue Inc.
5. NICODERM®Product License. February 16, 2016.
6. Fiore MC, Jaén CR, Baker TB, et al. Clinical Practice Guideline: Treating Tobacco Use and Dependence: 2008 Update.
7. Zhang B, et al. Duration of nicotine replacement therapy use and smoking cessation: A population-based longitudinal survey. Am J Epidemiol 2015;181:513-20.
8. Burns WK, Levinson AH. Discontinuation of nicotine replacement therapy among smoking-cessation attempters. Am J Prev Med 2008;34(3):212-15.
9. Siahpush M, Shaikh R, McCarthy M, et al. Association Between Duration of Use of Pharmacotherapy and Smoking Cessation from a National Survey. BMJ 2015;5:e006229.
10. World Health Organization. Proposal for inclusion on nicotine replacement therapy in the WHO Model List of Essential Medicines. 2009.
11. Schnoll RA, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med 2015;175(4):504-11.
12. Murray R, Bailey W, Daniels K, et al. Safety of Nicotine Polacrilex Gum Used by 3,094 Participants in the Lung Health Study*. Chest 1996;109:438–435.
13. McNeill A, Brose LS, Calder R, Bauld L & Robson D (2018). Evidence review of e-cigarettes and heated tobacco products 2018. A report commissioned by Public Health England. London: Public Health England.
14. Government of Canada. Concentration of Nicotine in Vaping Products Regulations (proposed Regulations) Regulatory impact analysis statement. Available at: http://www.gazette.gc.ca/rp-pr/p1/2020/2020-12-19/html/reg3-eng.html
15. Callahan-Lyon, P. Electronic cigarettes: Human health effects. Tobacco Control 2014;23:ii36-ii40.
16. Kaur G, Pinkston R, Mclemore B, et al. Immunological and toxicological risk assessment of e-cigerattes. Eur Respir Rev 2018;27(147):170119.