A study used the 28-question version of the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ), a validated, allergy-specific measurement of overall QOL which measures 7 domains:
Activities
Sleep
Nasal symptoms
Eye symptoms
Non-nose, Non-eye symptoms
Practical problems
Emotional
In seasonal allergic rhinitis, REACTINE® significantly improved adult patients’ QOL vs placebo
Demonstrated improvement in quality of life in adults with SAR measured by the RQLQ at week 1 and week 2
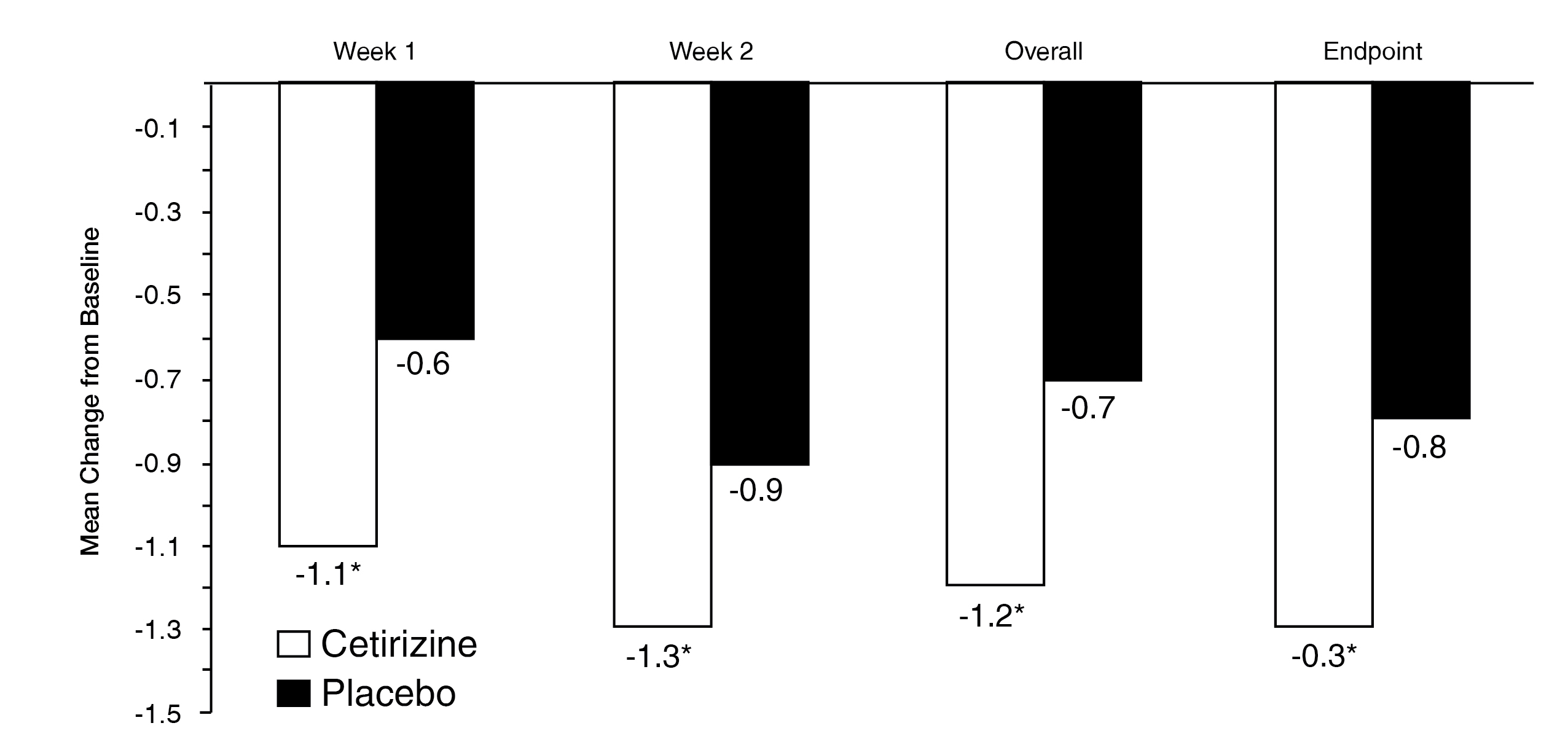
Change in baseline scores = 3.3 and 3.2 for REACTINE® and placebo, respectively. §P < 0.001.
Adapted from Murray, et al.
Demonstrated improvement in individual RQLQ domains at week 2
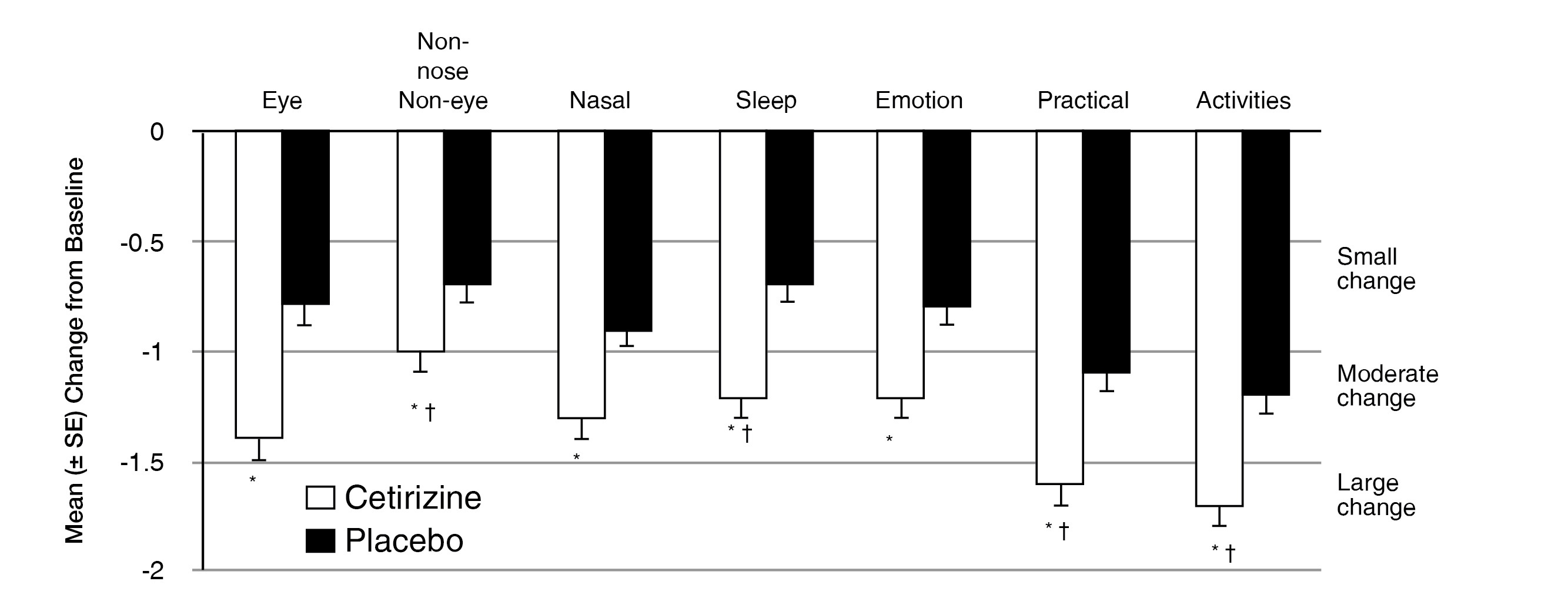
॥P < 0.001 vs placebo. ¶MID (minimally important difference). A change of ≥0.5 from baseline in RQLQ score defined as the smallest difference perceived by patients as being beneficial or clinically meaningful. A change of ≥1.0 and ≥1.5 from baseline represent a moderate and large change, respectively.
Adapted from Murray, et al.
REACTINE® reduced work impairment in adults with seasonal allergic rhinitis*‡
This study assessed the impact of allergy treatment on self-reported activity impairment using the Work Productivity and Activity Impairment-Allergy Specific (WPAI-AS) questionnaire, which measures performance based on‡:
Impairment at work/school (i.e., work hours missed, impairment at work, overall-work impairment, classroom hours missed, impairment in classroom, overall classroom impairment)
Activity impairment (i.e., limitations on regular daily activities such as shopping and child care)
Work productivity and activity impairment-allergy specific (WPAI-AS) domain score change at Week 2 *‡
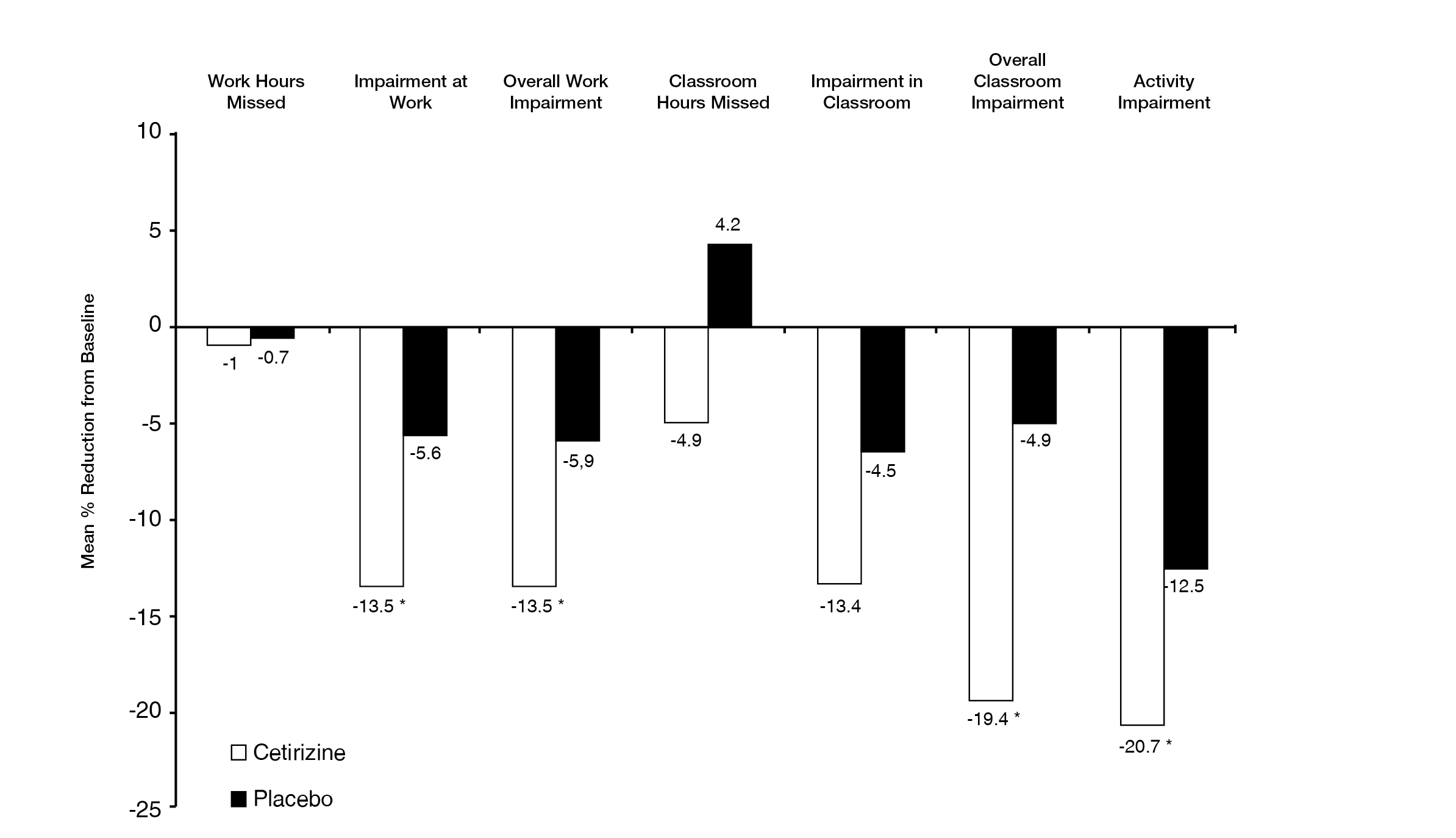
WPAI-AS=Work Productivity and Activity Impairment-Allergy Specific
**F-test p < 0.001 vs placebo.
Adapted from Murray, et al.
Footnotes
*Randomized, placebo-controlled, 3-week study of adult patients with seasonal allergic rhinitis (n=431 for each treatment group).
†Patient disease-specific quality of life was assessed using the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ), which measures 7 domains: activities, sleep, nasal symptoms, eye symptoms, non-nose, non-eye symptoms, practical problems, and emotional.
‡Impact on self-reported activity impairment was assessed using the Work Productivity and Activity Impairment-Allergy Specific (WPAI-AS) questionnaire, which measures performance based on impairment at work/school (i.e., work hours missed, impairment at work, overall-work impairment, classroom hours missed, impairment in classroom, overall classroom impairment) and activity impairment (i.e., limitations on regular daily activities such as shopping and child care)
References
1. Murray JJ, Nathan RA, Bronsky EA, Olufade AO, Chapman D, Kramer B. Comprehensive evaluation of cetirizine in the management of seasonal allergic rhinitis: impact on symptoms, quality of life, productivity, and activity impairment. Allergy Asthma Proc. 2002;23(6):392-398.
